
How is the pressure inside the test tube measured when the liquid reaches its boiling point?
The pressure in the test tube is the same as the barometric pressure in the room.
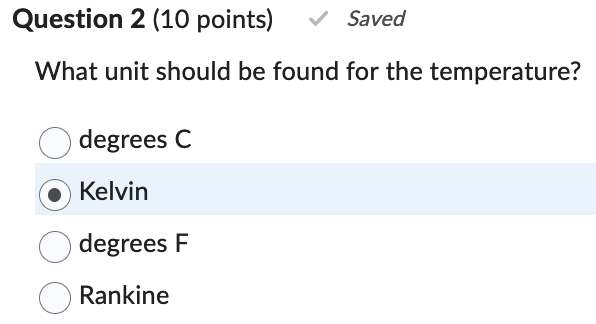
What unit should be found for the temperature?
Kelvin

During this experiment, it is necessary to solve for P2 in the Clausius-Clapeyron equation: ln(P2/P1) = ( delta H/R)[(1/T2)-(1/T1)]. Since the right hand side of the equation is able to be determined as a real number (we'll call "z"), the exercise becomes an algebraic problem of this form:
ln(a/b) = z
Screen reader note: ln left parenthesis a over b right parenthesis = z. End of note
Which formula allows for you to solve for the quantity "a"?
HINT: See http://www.sosmath.com/algebra/logs/log4/log47/log47.html for a review of solving logarithmic equations.
a = bez or a = b times e to the power of z

Where does the tip of the thermometer need to be when measuring the boiling point of the unknown liquid?
About half an inch above the level of the liquid.
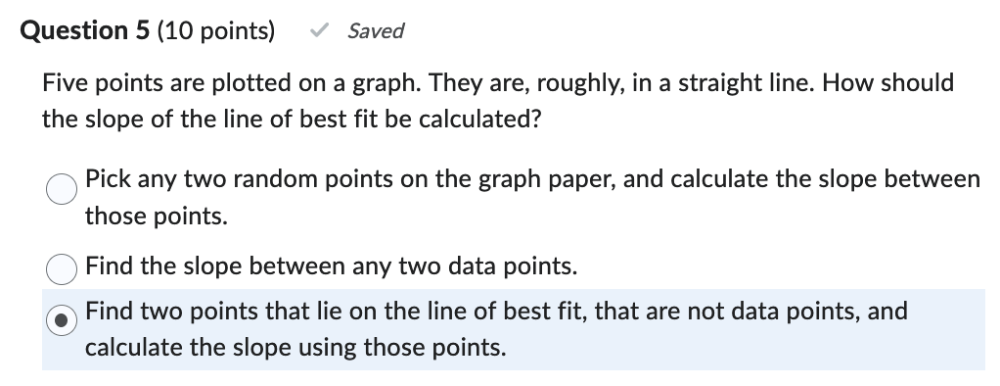
Five points are plotted on a graph. They are, roughly, in a straight line. How should the slope of the line of best fit be calculated?
Find two points that lie on the line of best fit, that are not data points, and calculate the slope using those points.