Write the full electron configuration for P. Put a space between each sublevel. Write superscripts as normal numbers (like 1s2 2s2 2p6 etc.)
1s2 2s2 2p6 3s2 3p3
Write the full electron configuration for Sr. Put a space between each sublevel. Write superscripts as normal numbers (like 1s2 2s2 2p6 etc.)
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2
Write the full electron configuration for Pb. Put a space between each sublevel. Write superscripts as normal numbers (like 1s2 2s2 2p6 etc.)
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p2
Write the full electron configuration for Fe+2. Put a space between each sublevel. Write superscripts as normal numbers (like 1s2 2s2 2p6 etc.)
Hint: Write out the complete electron configuration for the Fe atom first and then adjust for the charge.
1s2 2s2 2p6 3s2 3p6 3d6
Ex.
removed 4s2 from 1s2 2s2 2p6 3s2 3p6 4s2 3d6
What group of elements is represented by d block?
transition metals
Draw an energy level diagram for nitrogen. Start with the 1s orbital and use arrows to represent the electrons.
How many unpaired electrons does nitrogen have?
3
The number of unpaired electrons in Na is
1
The number of unpaired electrons in Br is
1
The number of unpaired electrons in S is
2
To which grouping does Cl belong?
halogens
To which grouping does P belong?
nonmetals
To which grouping does Na belong?
alkali metals
State the number of energy levels for As.
4
Ex.
Based off number of rows at the left of the periodic table.
State the number of energy levels for Br.
4
State the number of valence electrons for Se.
6
Ex.
Based off number of columns at top of the periodic table.
State the number of valence electrons for Al.
3
N is smaller than N3-.
True
Fe is larger than Fe2+.
True
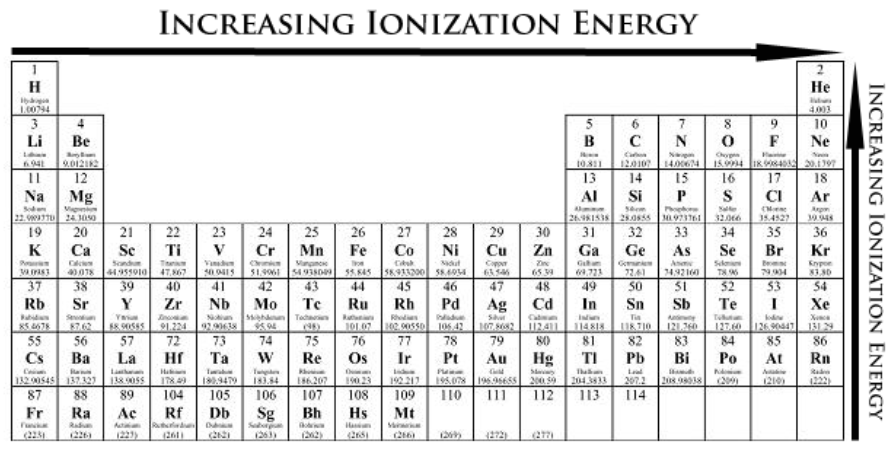
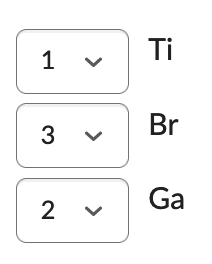
Order the following from highest ionization energy (1) to lowest ionization energy (3):
Ga, Ti, Br
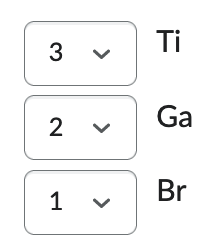
Order the following from lowest ionization energy (1) to highest ionization energy (3):
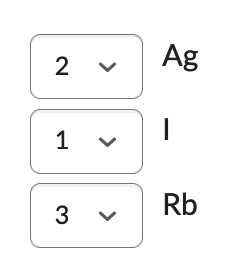
Ag, Rb, I
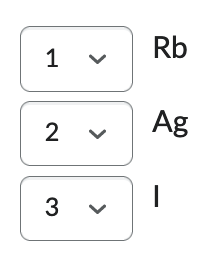
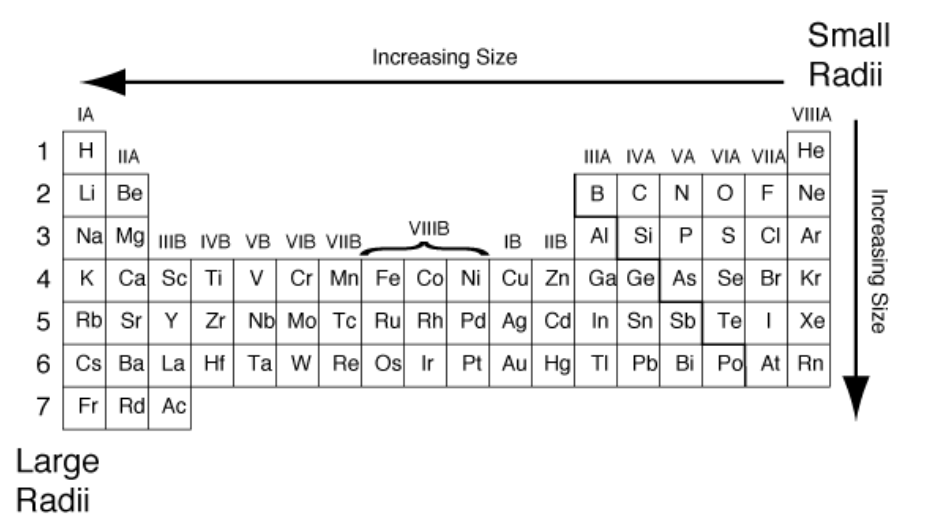
Order the following from smallest (1) to largest (3):
Ag, Rb, I
Order the following from largest (1) to smallest (3):
Ga, Ti, Br