Calculate the wavelength of light (in nm) if the light has a frequency of (4.60x10^14) s-1. Use a value of (3.0000x10^8) for the speed of light.
Remember 1 m = (1.000x10^9) nm
Do not include units with your answer.
Note: Your answer is assumed to be reduced to the highest power possible.
6.52 x 102
Calculate the wavelength of light (in nm) if the light has a frequency of (3.400x10^14) s-1. Use a value of (3.00x10^8) for the speed of light.
Remember 1 m = (1.0000x10^9) nm
Do not include units with your answer.
Note: Your answer is assumed to be reduced to the highest power possible.
8.82 x 102
Calculate the frequency of light (in s-1 also known as Hz) with a wavelength of (4.00x10^2) nm. Use a value of (3.0000x10^8) for the speed of light.
Remember 1 m = (1.000x10^9) nm
Do not include units with your answer.
Note: Your answer is assumed to be reduced to the highest power possible.
7.50 x 1014
Calculate the frequency of light (in s-1 also known as Hz) with a wavelength of (6.70x10^2) nm. Use a value of (3.0000x10^8) for the speed of light.
Remember 1 m = (1.000x10^9) nm
Do not include units with your answer.
Note: Your answer is assumed to be reduced to the highest power possible.
4.48 x 1014
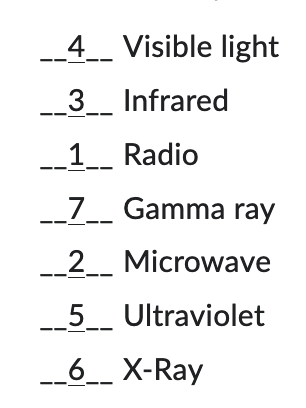
Rank the following types of radiation from lowest energy to highest energy.
1 being the lowest energy and 7 being the highest energy
Remember: Ephoton = hν
Calculate the energy of a photon of light (in Joules) with a wavelength of (6.1000x10^2) nm. Use a value of (3.00x10^8) for the speed of light.
Remember 1 m = (1.0000x10^9) nm
h = (6.6260x10^-34) Js
Do not include units with your answer.
Note: Your answer is assumed to be reduced to the highest power possible.
3.2587 x 10-19
Calculate the energy of a photon of light (in Joules) with a wavelength of (4.30x10^2) nm. Use a value of (3.000x10^8) for the speed of light.
Remember 1 m = (1.0000x10^9) nm
h = (6.6260x10^-34) Js
Do not include units with your answer.
Note: Your answer is assumed to be reduced to the highest power possible.
4.62 x 10-19
An electron transitions from the n = (5.0000x10^0) energy level to the n = (2.0000x10^0) energy. Calculate the energy, in Joules, associated with this energy transition. If the transition is exothermic be sure to include the appropriate sign.
Use the equation:
-------------------------------
This is for administrative purposes only.
Rydberg Constant = (2.18x10^-18)
Note: Your answer is assumed to be reduced to the highest power possible.
-4.5780 x 10-19
An electron transitions from the n = (5.00x10^0) energy level to the n = (2.000x10^0) energy. Calculate the energy, in Joules, associated with this energy transition. If the transition is exothermic be sure to include the appropriate sign.
Use the equation:
-------------------------------
This is for administrative purposes only.
Rydberg Constant = (2.1800x10^-18)
Note: Your answer is assumed to be reduced to the highest power possible.
-4.58 x 10-19
An s orbital has what shape?
sphere
A p orbital has what shape?
dumbbell
How many electrons does the s sublevel contain?
2 electrons
How many electrons can the p sublevel hold?
6 electrons
How many orbitals are in a d sublevel?
5 orbitals
Consider the list of quantum numbers below:
n = 4; l = 1; ml = -1; ms = +1/2
(OR n = 4; l = 1; m sub l = minus 1; m sub s
= +1/2)
In which orbital subshell will this electron be located?
4p
Consider the list of quantum numbers below:
n = 4; l = 1; ml = -1; ms = +1/2
(OR n = 4; l = 1; m sub l = minus 1; m sub s
= +1/2)
Is this a valid set of quantum numbers?
- Yes
- No
Yes
Consider the list of quantum numbers below:
n = 6; l = 1; ml = 0; ms = -1/2
(OR n = 6; l = 1; m sub l = 0; m sub s =
minus 1/2)
In which orbital subshell will this electron be located?
6p
Consider the list of quantum numbers below:
n = 4; l = 2; ml = -3; ms= -1/2
(OR n = 4; l = 2; m sub l = minus 3; m sub s
= minus 1/2)
Is this a valid set of quantum numbers?
- Yes
- No
No
An electron is located in the 5d orbital. Quantum numbers for this electron would be: <p>n = ______</p> <p>l = ______</p>
n = ___ 5 ___
l = ___ 2 ___