About how much water should be used to calibrate the eudiometer tube?
- 10 mL
- 20 mL
- 40 mL
- 50 mL
- 30 mL
40 mL
How will the conversion factor "f" for eudiometer tube (ET) calibration be calculated?
Real volume from buret/Reading from ET
What is the mole ratio of magnesium to hydrogen gas in the reaction used in this experiment?
1:1

A eudiometer tube (ET) is found to have a conversion factor "f" of 0.81 mL/ET unit. The experimental procedure for this lab is followed and produces a reading on the ET tube of 40.40 mL. What is the actual volume of hydrogen gas in the ET (in Liters)? Report the answer to 4 significant figures.
0.81 x 40.40 x (1 L / 1000 mL) = 0.03272 L
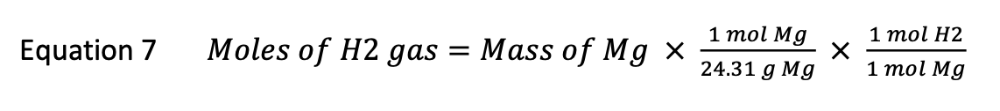
How many moles of hydrogen gas are generated by 0.038 grams of magnesium? Report the answer to four significant figures. DO NOT USE EXPONENTS.
0.038 g Mg x ( 1 mol Mg / 24.31 g Mg ) x ( 1 mol H2 / 1 mol Mg ) = 0.001563 mol