In general, the hydrolysis of ATP drives cellular work by __________.
- changing to ADP and phosphate
- releasing free energy that can be coupled to other reactions
- acting as a catalyst
- releasing heat
- lowering the free energy of the reaction
releasing free energy that can be coupled to other reactions
Ex.
In general, the hydrolysis of ATP drives cellular work by releasing free energy that can be coupled to other reactions.
Energy coupling is key feature in the way cells manage their energy resources to do work, which is the use of an exergonic process to drive an endergonic one. ATP is responsible for mediating most energy coupling in cells, and in most cases it acts as the immediate source of energy (not a catalyst) that powers cellular work. Byproducts such as heat are not effective energy sources for such work.
Which of the following statements about the active site of an enzyme is correct?
- Coenzymes are rarely found in the active site of an enzyme.
- The active site has a fixed structure (shape).
- The active site may resemble a groove or pocket in the surface of a protein into which the substrate fits.
- The active site allows the reaction to occur under the same environmental conditions as the reaction without the enzyme.
- The structure of the active site is not affected by changes in temperature.
The active site may resemble a groove or pocket in the surface of a protein into which the substrate fits.
Ex.
The active site may resemble a groove or pocket in the surface of a protein into which the substrate fits is correct.
Only a restricted region of the enzyme molecule actually binds to the substrate. This region, called the active site, is typically a pocket or groove on the surface of the enzyme where catalysis occurs. Usually, the active site is formed by only a few of the enzyme’s amino acids, with the rest of the protein molecule providing a framework that determines the configuration of the active site. The specificity of an enzyme is attributed to a compatible fit between the shape of its active site and the shape of the substrate.
An enzyme is not a stiff structure locked into a given shape. In fact, recent work by biochemists has shown clearly that enzymes (and other proteins as well) seem to “dance” between subtly different shapes in a dynamic equilibrium, with slight differences in free energy for each “pose.”
Many enzymes require nonprotein helpers for catalytic activity. These adjuncts, called cofactors, may be bound tightly to the enzyme as permanent residents, or they may bind loosely and reversibly along with the substrate. The cofactors of some enzymes are inorganic, such as the metal atoms zinc, iron, and copper in ionic form. If the cofactor is an organic molecule, it is more specifically called a coenzyme. Most vitamins are important in nutrition because they act as coenzymes or raw materials from which coenzymes are made.
The shape that best fits the substrate isn’t necessarily the one with the lowest energy, but during the very short time the enzyme takes on this shape, its active site can bind to the substrate. It has been known for more than 50 years that the active site itself is also not a rigid receptacle for the substrate.
Which of these statements about enzyme inhibitors is true?
- When the product of an enzyme or an enzyme sequence acts as its inhibitor, this is known as positive feedback.
- A competitive inhibitor binds to the enzyme at a place that is separate from the active site.
- The action of inhibitors may be reversible or irreversible.
- A noncompetitive inhibitor does not change the shape of the active site.
- Inhibition of enzyme function by compounds that are not substrates is something that only occurs under controlled conditions in the laboratory.
The action of inhibitors may be reversible or irreversible.
Ex.
The action of inhibitors may be reversible or irreversible is true.
Certain chemicals selectively inhibit the action of specific enzymes, and we have learned a lot about enzyme function by studying the effects of these molecules. If the inhibitor attaches to the enzyme by covalent bonds, inhibition is usually irreversible. Many enzyme inhibitors, however, bind to the enzyme by weak interactions, in which case inhibition is reversible.
Some reversible inhibitors resemble the normal substrate molecule and compete for admission into the active site. These mimics, called competitive inhibitors, reduce the productivity of enzymes by blocking substrates from entering active sites. This kind of inhibition can be overcome by increasing the concentration of substrate so that as active sites become available, more substrate molecules than inhibitor molecules are around to gain entry to the sites.
In contrast, noncompetitive inhibitors do not directly compete with the substrate to bind to the enzyme at the active site. Instead, they impede enzymatic reactions by binding to another part of the enzyme. This interaction causes the enzyme molecule to change its shape in such a way that the active site becomes less effective at catalyzing the conversion of substrate to product.
Which of the following statements is correct regarding kinetic and potential energy?
- Potential energy is related to the relative motion of objects, and kinetic energy is the energy that matter possesses because of its location or structure.
- None of the listed responses is correct.
- Chemical energy is a type of kinetic energy, and thermal energy is a type of potential energy.
- Kinetic energy is associated with the relative motion of objects, and potential energy is the energy that matter possesses because of its location or structure.
- Potential energy cannot be converted to kinetic energy.
Kinetic energy is associated with the relative motion of objects, and potential energy is the energy that matter possesses because of its location or structure.
Ex.
Kinetic energy is associated with the relative motion of objects, and potential energy is the energy that matter possesses because of its location or structure.
Energy is the capacity to cause change. In everyday life, energy is important because some forms of energy can be used to do work—that is, to move matter against opposing forces, such as gravity and friction. Put another way, energy is the ability to rearrange a collection of matter. Energy exists in various forms, and the work of life depends on the ability of cells to transform energy from one form to another. Energy can be associated with the relative motion of objects; this energy is called kinetic energy . Moving objects can perform work by imparting motion to other matter. Energy that is not kinetic is called potential energy ; it is the energy that matter possesses because of its location or structure.
Potential energy is the energy of position or structure while kinetic energy is related to the energy of motion.
Potential energy can be converted to kinetic energy.
Chemical energy is a type of potential energy, and thermal energy is a type of kinetic energy.
Which of the following statements is correct regarding ATP?
- ATP cannot transfer energy to other molecules.
- The energy in an ATP molecule is released from the adenine group.
- The energy in an ATP molecule is released from the ribose group.
- ATP molecules do not release free energy when hydrolyzed.
- The energy in an ATP molecule is released through hydrolysis of one of the phosphate groups.
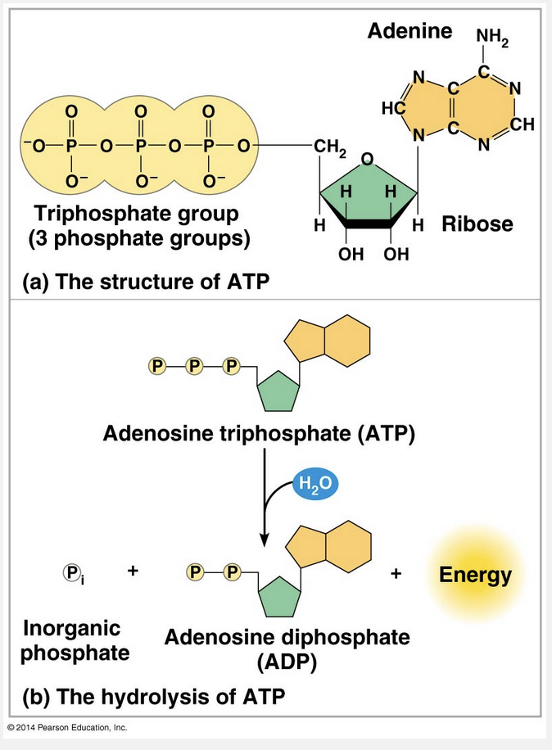
The energy in an ATP molecule is released through hydrolysis of one of the phosphate groups.
Ex.
The energy in an ATP molecule is released through hydrolysis of one of the phosphate groups.
ATP (adenosine triphosphate) is responsible for mediating most energy coupling in cells, and in most cases it acts as the immediate source of energy that powers cellular work. ATP contains the sugar ribose, with the nitrogenous base adenine and a chain of three phosphate groups (the triphosphate group) bonded to it. The bonds between the phosphate groups of ATP can be broken by hydrolysis. When the terminal phosphate bond is broken by the addition of a water molecule, a molecule of inorganic phosphate leaves the ATP, which becomes adenosine diphosphate, or ADP. The reaction is exergonic and releases 7.3 kcal of energy per mole of ATP hydrolyzed.
The energy released from an ATP molecule is based on the hydrolysis of one of the phosphate groups, not the adenine.
The energy released from an ATP molecule is based on the hydrolysis of one of the phosphate groups, not the ribose.
The hydrolysis of ATP releases free energy.
ATP can transfer energy to other molecules through the transfer of one of the phosphate groups.
Which of the following statements about enzyme function is correct?
- None of the listed responses is correct.
- Enzymes can greatly speed up reactions, but they cannot change the net energy output because they cannot change the activation energy.
- Enzymes can change the equilibrium point of reactions, but they cannot speed up reactions because they cannot change the net energy output.
- Enzymes can lower the activation energy of reactions, but they cannot change the equilibrium point because they cannot change the net energy output.
- Enzymes can greatly speed up reactions, but they cannot change the activation energy because they cannot change the net energy output.
Enzymes can lower the activation energy of reactions, but they cannot change the equilibrium point because they cannot change the net energy output.
Ex.
Enzymes can lower the activation energy of reactions, but they cannot change the equilibrium point because they cannot change the net energy output.
An enzyme catalyzes a reaction by lowering the activation energy (EA) barrier, enabling the reactant molecules to absorb enough energy to reach the transition state even at moderate temperatures. An enzyme cannot change the ΔG for a reaction; it cannot make an endergonic reaction exergonic.
Enzymes can only hasten reactions that would eventually occur anyway, but this function makes it possible for the cell to have a dynamic metabolism, routing chemicals smoothly through the cell’s metabolic pathways. And because enzymes are very specific for the reactions they catalyze, they determine which chemical processes will be going on in the cell at any particular time.
Most metabolic reactions are reversible, and an enzyme can catalyze either the forward or the reverse reaction, depending on which direction has a negative ΔG. This in turn depends mainly on the relative concentrations of reactants and products. The net effect is always in the direction of equilibrium, a term that describes a state of maximum stability.
An exergonic (spontaneous) reaction is a chemical reaction that __________.
- cannot occur outside of a living cell
- releases energy when proceeding in the forward direction
- leads to a decrease in the entropy of the universe
- is common in anabolic pathways
- occurs only when an enzyme or other catalyst is present
releases energy when proceeding in the forward direction
Ex.
An exergonic (spontaneous) reaction is a chemical reaction that releases energy when proceeding in the forward direction.
Based on their free-energy changes, chemical reactions can be classified as either exergonic (“energy outward”) or endergonic (“energy inward”). An exergonic reaction proceeds with a net release of free energy (ΔG < 0). From the equation ΔG = ΔH – TΔS, for ΔG to be negative then either ΔH must be negative (the system gives up enthalpy and H decreases) or TΔS must be positive (the system gives up order and S increases), or both. More than a century of experiments has shown that only processes with a negative ΔG are spontaneous. When ΔH and TΔS are tallied, ΔG has a negative value (ΔG < 0) for all spontaneous processes. In other words, every spontaneous process decreases the system’s free energy, and processes that have a positive or zero ΔG are never spontaneous.
At low temperatures, a particular enzyme catalyzes a reaction, but at a slow rate. At high temperatures, the enzyme is completely inactive. What statement best explains the difference in how temperature affects the function of this enzyme?
- Temperature has no effect on enzyme function.
- The enzyme functions best at both low and high temperatures.
- High temperature provides the optimal environment in which this enzyme functions.
- Low temperatures cause the enzyme to denature, and high temperatures cause the enzyme to move too fast to bind to its substrate.
- At low temperatures, there is not enough free energy for the enzyme to function at a high rate, and at high temperatures, the enzyme is denatured, leaving it nonfunctional.
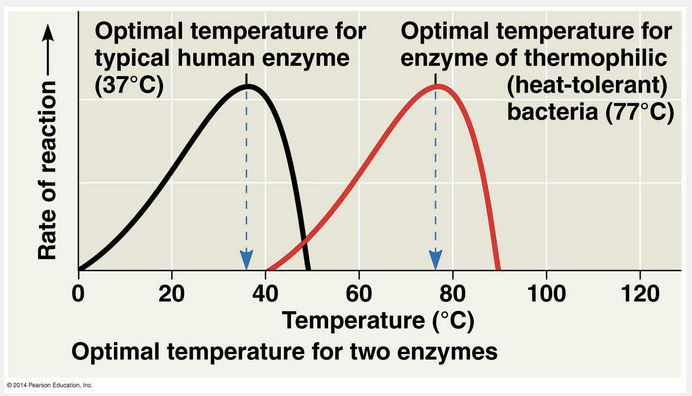
At low temperatures, there is not enough free energy for the enzyme to function at a high rate, and at high temperatures, the enzyme is denatured, leaving it nonfunctional.
Ex.
At low temperatures, there is not enough free energy for the enzyme to function at a high rate, and at high temperatures, the enzyme is denatured, leaving it nonfunctional.
The activity of an enzyme—how efficiently it functions—is affected by general environmental factors, such as temperature and pH. The three-dimensional structures of proteins are sensitive to their environment. As a consequence, each enzyme works better under some conditions than under other conditions because these optimal conditions favor the most active shape of the enzyme. Temperature and pH are environmental factors important to the activity of an enzyme. Up to a point, the rate of an enzymatic reaction increases with increasing temperature, partly because substrates collide with active sites more frequently when the molecules move rapidly. Above that temperature, however, the speed of the enzymatic reaction drops sharply. The thermal agitation of the enzyme molecule disrupts the hydrogen bonds, ionic bonds, and other weak interactions that stabilize the active shape of the enzyme, and the protein molecule eventually denatures. Each enzyme has an optimal temperature at which its reaction rate is greatest.
Low temperatures do not usually cause the denaturing of an enzyme. High temperatures can speed up enzymatic reactions, but only to the point where the enzyme begins to lose its structure and be denatured.
Since the enzyme exhibits little function at extreme temperatures, it is more likely to function better at moderate temperatures.
Since the enzyme’s function is different in both conditions, temperature appears to alter its function.
High temperatures can speed up enzymatic reactions, but only to the point where the enzyme begins to lose its structure and be denatured.
Which of the following correctly states the relationship between anabolic and catabolic pathways?
- Catabolic pathways produce usable cellular energy by synthesizing more complex organic molecules.
- Degradation of organic molecules by anabolic pathways provides the energy to drive catabolic pathways.
- Energy derived from catabolic pathways is used to drive the breakdown of organic molecules in anabolic pathways.
- The flow of energy between catabolic and anabolic pathways is reversible.
- Anabolic pathways synthesize more complex organic molecules using the energy derived from catabolic pathways.
Anabolic pathways synthesize more complex organic molecules using the energy derived from catabolic pathways.
Ex.
Anabolic pathways synthesize more complex organic molecules using the energy derived from catabolic pathways.
Metabolism as a whole manages the material and energy resources of the cell. Some metabolic pathways release energy by breaking down complex molecules to simpler compounds. These degradative processes are called catabolic pathways, or breakdown pathways. A major pathway of catabolism is cellular respiration, in which the sugar glucose and other organic fuels are broken down in the presence of oxygen to carbon dioxide and water. Energy that was stored in the organic molecules becomes available to do the work of the cell, such as ciliary beating or membrane transport.
Anabolic pathways, in contrast, consume energy to build complicated molecules from simpler ones; they are sometimes called biosynthetic pathways. Examples of anabolism are the synthesis of an amino acid from simpler molecules and the synthesis of a protein from amino acids. Catabolic and anabolic pathways are the “downhill” and “uphill” avenues of the metabolic landscape. Energy released from the downhill reactions of catabolic pathways can be stored and then used to drive the uphill reactions of anabolic pathways.
ATP allosterically inhibits enzymes in ATP-producing pathways. The result of this is called __________.
- competitive inhibition
- positive feedback
- denaturing
- cooperativity
- feedback inhibition
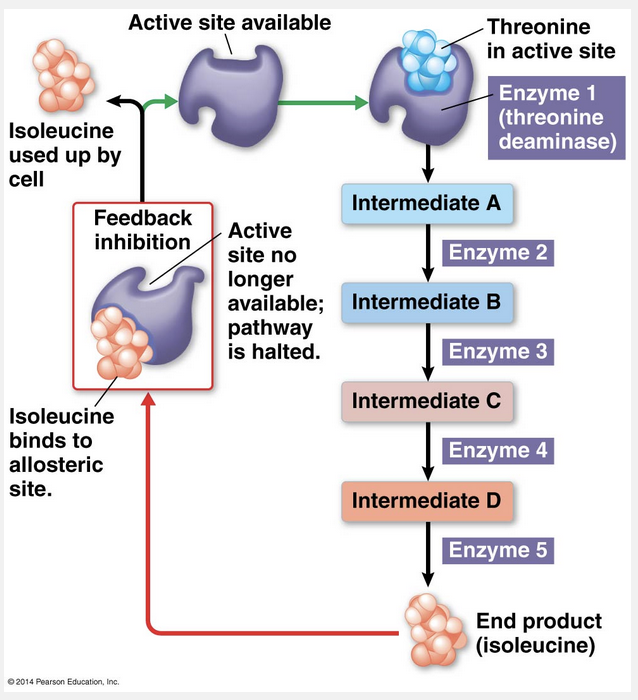
feedback inhibition
Ex.
ATP inhibits ATP-producing pathways through feedback inhibition.
When ATP allosterically inhibits an enzyme in an ATP-generating pathway, the result is feedback inhibition, a common mode of metabolic control. In feedback inhibition, a metabolic pathway is halted by the inhibitory binding of its end product to an enzyme that acts early in the pathway. Feedback inhibition thereby prevents the cell from making more product than is necessary and thus wasting chemical resources.
Positive feedback is a type of feedback where the product facilitates, but does not inhibit, a process.
ATP is an allosteric inhibitor, not a competitive inhibitor.
Cooperativity is a mechanism that amplifies the response of enzymes to substrates.
Denaturing is the breakdown in structure of an enzyme, not an inhibition process.
Which of the following is changed by the presence of an enzyme in a reaction?
- The G value for the reactants
- The activation energy
- The sign of ΔG
- The G value for the products
- The magnitude of ΔG
The activation energy
Ex.
The activation energy is changed by the presence of an enzyme in a reaction.
An enzyme is a macromolecule that acts as a catalyst, a chemical agent that speeds up a reaction without being consumed by the reaction. An enzyme catalyzes a reaction by lowering the activation energy (EA) barrier, enabling the reactant molecules to absorb enough energy to reach the transition state even at moderate temperatures. An enzyme cannot change the ΔG for a reaction; it cannot make an endergonic reaction exergonic. Enzymes can only hasten reactions that would eventually occur anyway, but this function makes it possible for the cell to have a dynamic metabolism, routing chemicals smoothly through the cell’s metabolic pathways. And because enzymes are very specific for the reactions they catalyze, they determine which chemical processes will be going on in the cell at any particular time.
If the entropy of a living organism is decreasing, which of the following is most likely to be occurring simultaneously?
- The first law of thermodynamics is being violated.
- Energy input into the organism must be occurring to drive the decrease in entropy.
- In this situation, the second law of thermodynamics must not apply.
- The entropy of the organism's environment must also be decreasing.
- Heat is being used by the organism as a source of energy.
Energy input into the organism must be occurring to drive the decrease in entropy.
Ex.
If the entropy of a living organism is decreasing, then energy input into the organism must be occurring to drive the decrease in entropy.
A logical consequence of the loss of usable energy during energy transfer or transformation is that each such event makes the universe more disordered. Scientists use a quantity called entropy as a measure of disorder, or randomness. The more randomly arranged a collection of matter is, the greater its entropy. The second law of thermodynamics states that every energy transfer or transformation increases the entropy of the universe. Although order can increase locally, there is an unstoppable trend toward randomization of the universe as a whole. Living systems increase the entropy of their surroundings, as predicted by thermodynamic law. It is true that cells create ordered structures from less organized starting materials. For example, simpler molecules are ordered into the more complex structure of an amino acid, and amino acids are ordered into polypeptide chains. At the organismal level as well, complex and beautifully ordered structures result from biological processes that use simpler starting materials. However, an organism also takes in organized forms of matter and energy from the surroundings and replaces them with less ordered forms. For example, an animal obtains starch, proteins, and other complex molecules from the food it eats. As catabolic pathways break these molecules down, the animal releases carbon dioxide and water—small molecules that possess less chemical energy than the food did. The depletion of chemical energy is accounted for by heat generated during metabolism. On a larger scale, energy flows into most ecosystems in the form of light and exits in the form of heat.
What best characterizes the role of ATP in cellular metabolism?
- It is catabolized to carbon dioxide and water.
- The charge on the phosphate group of ATP tends to make the molecule very water-soluble.
- The free energy released by ATP hydrolysis that may be coupled to an endergonic process via the formation of a phosphorylated intermediate.
- The ΔG associated with its hydrolysis is positive.
- The release of free energy during the hydrolysis of ATP heats the surrounding environment.
The free energy released by ATP hydrolysis that may be coupled to an endergonic process via the formation of a phosphorylated intermediate.
Ex.
The role of ATP in cellular metabolism can best be characterized as the free energy released by ATP hydrolysis that may be coupled to an endergonic process via the formation of a phosphorylated intermediate.
For example, with the help of specific enzymes, the cell is able to use the energy released by ATP hydrolysis directly to drive chemical reactions that, by themselves, are endergonic. If the ΔG of an endergonic reaction is less than the amount of energy released by ATP hydrolysis, then the two reactions can be coupled so that, overall, the coupled reactions are exergonic. This usually involves the transfer of a phosphate group from ATP to some other molecule, such as the reactant. The recipient with the phosphate group covalently bonded to it is then called a phosphorylated intermediate. The key to coupling exergonic and endergonic reactions is the formation of this phosphorylated intermediate, which is more reactive (less stable) than the original unphosphorylated molecule.
Which of the following statements correctly describes cofactors and coenzymes?
- Both cofactors and coenzymes act as allosteric inhibitors to various enzymes.
- Neither cofactors nor coenzymes assist enzyme function.
- Cofactors that are metal ions activate enzymes, but coenzymes deactivate them.
- Both are nonprotein enzyme helpers; but most coenzymes are metal ions, and most cofactors are organic molecules.
- Both are nonprotein enzyme helpers; but most cofactors are metal ions, and coenzymes are organic molecules that are a specific type of cofactor.
Both are nonprotein enzyme helpers; but most cofactors are metal ions, and coenzymes are organic molecules that are a specific type of cofactor.
Ex.
Both are nonprotein enzyme helpers; but most cofactors are metal ions, and coenzymes are organic molecules that are a specific type of cofactor.
Many enzymes require nonprotein helpers for catalytic activity. These adjuncts, called cofactors, may be bound tightly to the enzyme as permanent residents, or they may bind loosely and reversibly along with the substrate. The cofactors of some enzymes, such as the metal atoms zinc, iron, and copper in ionic form, are inorganic. If the cofactor is an organic molecule, it is referred to more specifically as a coenzyme. Most vitamins are important in nutrition because they act as coenzymes or raw materials from which coenzymes are made.
Both cofactors and coenzymes are nonprotein enzyme helpers; but most cofactors are metal ions, and coenzymes are usually organic molecules.
Both cofactors and coenzymes help facilitate enzyme function.
Neither cofactors nor coenzymes act as allosteric inhibitors.
Cofactors do facilitate enzyme function, but coenzymes do not deactivate enzymes.
The primary manner in which cells manage their energy resources in order to do work is called energy coupling. Which of the following statements accurately defines energy coupling?
- Endergonic reactions drive exergonic reactions.
- Chemical reactions in cells are always at equilibrium.
- Endergonic and exergonic reactions occur independently of each other.
- Exergonic reactions drive endergonic reactions.
- Anabolic reactions drive catabolic reactions.
Exergonic reactions drive endergonic reactions.
Ex.
Exergonic reactions drive endergonic reactions.
Based on their free-energy changes, chemical reactions can be classified as either exergonic (“energy outward”) or endergonic (“energy inward”). An exergonic reaction proceeds with a net release of free energy. An endergonic reaction absorbs free energy from its surroundings. A key feature in the way cells manage their energy resources in order to do this work is energy coupling, the use of an exergonic process to drive an endergonic one. ATP is responsible for mediating most energy coupling in cells, and in most cases it acts as the immediate source of energy that powers cellular work.
Catabolic reactions typically drive anabolic reactions.
Exergonic reactions drive endergonic reactions.
If chemical reactions in cells are at equilibrium, the cell is dead.
Endergonic and exergonic reactions often occur together.
An exergonic reaction __________ free energy, and an endergonic reaction __________ free energy.
- absorbs; releases
- destroys; creates
- releases; releases
- releases; absorbs
- absorbs; absorbs
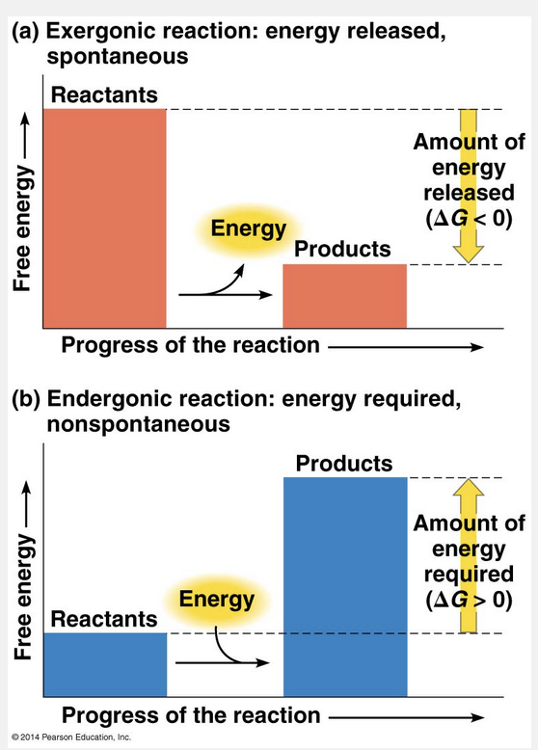
releases; absorbs
Ex.
An exergonic reaction releases free energy, and an endergonic reaction absorbs free energy.
Based on their free-energy changes, chemical reactions can be classified as either exergonic (“energy outward”) or endergonic (“energy inward”). An exergonic reaction proceeds with a net release of free energy. An endergonic reaction absorbs free energy from its surroundings.
Exergonic reactions release free energy, and endergonic reactions absorb free energy.
Energy cannot be created or destroyed.
Which of the following states the relevance of the first law of thermodynamics to biology?
- Photosynthetic organisms produce energy in sugars from sunlight.
- Energy can be freely transformed among different forms as long as the total energy is conserved.
- The total energy taken in by an organism must be greater than the total energy stored or released by the organism.
- Living organisms must increase the entropy of their surroundings.
- Energy is destroyed as glucose is broken down during cellular respiration.
Energy can be freely transformed among different forms as long as the total energy is conserved.
Ex.
The following states the relevance of the first law of thermodynamics to biology: Energy can be freely transformed among different forms as long as the total energy is conserved.
The first law of thermodynamics is relevant to biology because acquiring and using energy are necessary tasks for survival. The first law of thermodynamics, the energy of the universe is constant: Energy can be transferred and transformed, but it cannot be created or destroyed. The first law is also known as the principle of conservation of energy. For example, the electric company does not make energy, it merely converts one form of energy to another that is more convenient for us to use. By converting sunlight to chemical energy, a plant acts as an energy transformer, not an energy producer.
Cells use ATP constantly, but ATP is considered a renewable resource. What process makes this possible?
- ADP is generated by the addition of a phosphate group to ATP.
- None of the listed responses is correct.
- The hydrolysis of ATP is an irreversible reaction.
- ADP and ATP are stored in large amounts in a cell.
- ATP can be regenerated by the addition of a phosphate group to ADP.
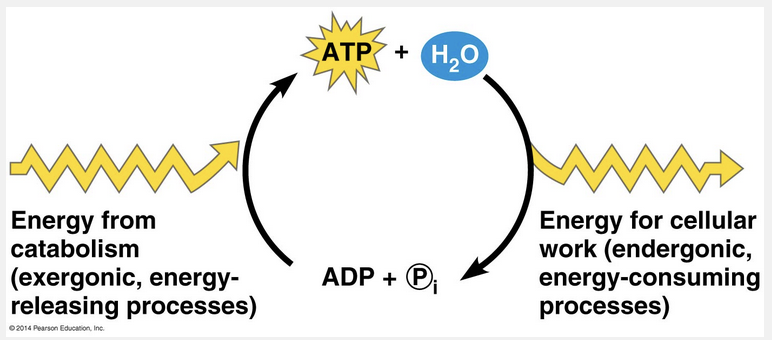
ATP can be regenerated by the addition of a phosphate group to ADP.
Ex.
ATP can be regenerated by the addition of a phosphate group to ADP.
An organism at work uses ATP continuously, but ATP is a renewable resource that can be regenerated by the addition of phosphate to ADP. The free energy required to phosphorylate ADP comes from exergonic breakdown reactions (catabolism) in the cell. This shuttling of inorganic phosphate and energy is called the ATP cycle, and it couples the cell’s energy-yielding (exergonic) processes to the energy-consuming (endergonic) ones.
Cells do not store large amounts of either ATP or ADP.
The hydrolysis of ATP is a reversible reaction.
ATP is generated by the addition of a phosphate group to ADP, not the opposite.
Which of the following statements is correct regarding competitive and noncompetitive enzyme inhibitors?
- Competitive inhibitors bind to the active site of an enzyme while noncompetitive inhibitors bind to an enzyme away from the active site.
- Neither type of inhibitor affects enzyme function.
- Inhibitors always bind irreversibly to an enzyme.
- Only competitive inhibitors affect enzyme function.
- Competitive inhibitors do not bind directly to the active site of an enzyme while noncompetitive inhibitors do.
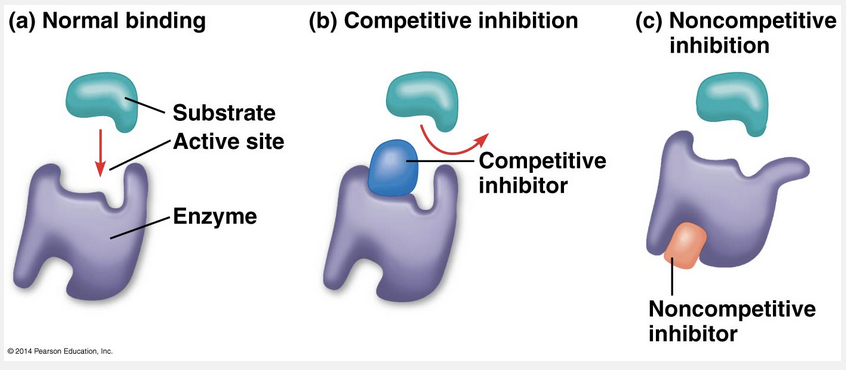
Competitive inhibitors bind to the active site of an enzyme while noncompetitive inhibitors bind to an enzyme away from the active site.
Ex.
Competitive inhibitors bind to the active site of an enzyme while noncompetitive inhibitors bind to an enzyme away from the active site.
Certain chemicals selectively inhibit the action of certain enzymes. Sometimes, the inhibitor attaches to the enzyme by covalent bonds, in which case the inhibition is usually irreversible. Many enzyme inhibitors, however, bind to the enzyme by weak interactions, and when this occurs the inhibition is reversible. Some reversible inhibitors resemble the normal substrate molecule and compete for admission into the active site. These mimics, called competitive inhibitors, reduce the productivity of enzymes by blocking substrates from entering active sites. This kind of inhibition can be overcome by increasing the concentration of substrate so that as active sites become available, more substrate molecules than inhibitor molecules are around to gain entry to the sites. In contrast, noncompetitive inhibitors do not directly compete with the substrate to bind to the enzyme at the active site. Instead, they impede enzymatic reactions by binding to another part of the enzyme. This interaction causes the enzyme molecule to change its shape in such a way that the active site becomes less effective at catalyzing the conversion of substrate to product.
Competitive inhibitors bind to the active site of an enzyme, and noncompetitive inhibitors bind to a site away from the active site.
Both types of inhibitors affect enzyme function.
Many inhibitors can bind reversibly to enzymes.
Which of the following statements about enzymes is true?
- The more heat that is added to a reaction, the faster the enzymes will function.
- All of the listed responses are correct.
- Enzymes speed up the rate of the reaction without changing the ΔG for the reaction.
- Enzymes react with their substrate (form chemical bonds), forming an enzyme-substrate complex, which irreversibly alters the enzyme.
- Enzymes increase the rate of a reaction by raising the activation energy for reactions.
Enzymes speed up the rate of the reaction without changing the ΔG for the reaction.
Ex.
Enzymes speed up the rate of the reaction without changing the ΔG for the reaction is true about enzymes.
An enzyme is a macromolecule that acts as a catalyst, a chemical agent that speeds up a reaction without being consumed by the reaction. An enzyme catalyzes a reaction by lowering the activation energy EA barrier, enabling the reactant molecules to absorb enough energy to reach the transition state even at moderate temperatures.
An enzyme cannot change the ΔG for a reaction; it cannot make an endergonic reaction exergonic. Enzymes can only hasten reactions that would eventually occur anyway, but this function makes it possible for the cell to have a dynamic metabolism, routing chemicals smoothly through the cell’s metabolic pathways. And because enzymes are very specific for the reactions they catalyze, they determine which chemical processes will be going on in the cell at any particular time.
Although heat can speed a reaction by allowing reactants to attain the transition state more often, this solution would be inappropriate for biological systems. First, high temperature denatures enzymes, which are proteins, and kills cells. Second, heat would speed up all reactions, not just those that are needed. Instead of heat, organisms use enzymes to speed up reactions.
Which of the following is an example of the second law of thermodynamics as it applies to biological reactions?
- All of the listed responses are correct.
- The aerobic respiration of one molecule of glucose produces six molecules each of carbon dioxide and water.
- Cellular respiration releases some energy as heat.
- None of the listed responses is correct.
- All types of cellular respiration produce ATP.
The aerobic respiration of one molecule of glucose produces six molecules each of carbon dioxide and water.
Ex.
An example of the second law of thermodynamics, as it applies to biological reactions, is the aerobic respiration of one molecule of glucose to produce six molecules of carbon dioxide and water.
The second law of thermodynamics states that spontaneous processes, those requiring no outside input of energy, increase the entropy (disorder) of the universe. In this example, carbon dioxide and water are more disordered than glucose. Cellular respiration, in which the sugar glucose and other organic fuels are broken down in the presence of oxygen to release energy and produce carbon dioxide and water, is a major pathway of catabolism. Energy that was stored in the organic molecules becomes available to do the work of the cell, such as ciliary beating or membrane transport.
As ATP begins to build up in a cell, metabolism slows down. How does this happen?
- None of the listed responses is correct.
- ATP acts as an allosteric inhibitor to many of the enzymes involved in metabolism, thus slowing their function.
- ATP binds to the active sites of many of the enzymes involved in metabolism, causing them to stop functioning.
- Excess ATP causes many of the enzymes involved in metabolism to denature.
- ATP acts as an activator, increasing the rate of its production.
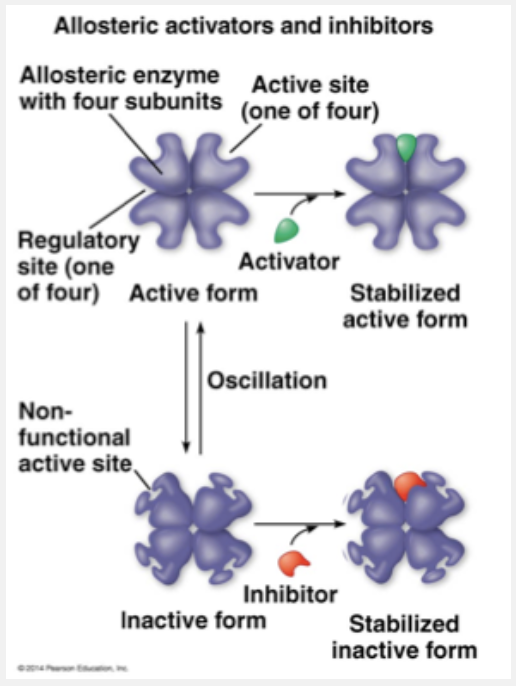
ATP acts as an allosteric inhibitor to many of the enzymes involved in metabolism, thus slowing their function.
Ex.
ATP acts as an allosteric inhibitor to many of the enzymes involved in metabolism, thus slowing their function.
Chemical chaos would result if all of a cell’s metabolic pathways were operating simultaneously. Intrinsic to life’s processes is a cell’s ability to tightly regulate its metabolic pathways by controlling when and where its various enzymes are active. It does this either by switching on and off the genes that encode specific enzymes or by regulating the activity of enzymes once they are made. Most enzymes known to be allosterically regulated are constructed from two or more subunits, each composed of a polypeptide chain with its own active site. The entire complex oscillates between two different shapes, one catalytically active and the other inactive. Fluctuating concentrations of regulators can cause a sophisticated pattern of responses in the activity of cellular enzymes. The products of ATP hydrolysis, for example, play a complex role in balancing the flow of traffic between anabolic and catabolic pathways because of their effects on key enzymes. ATP binds to several catabolic enzymes allosterically, lowering their affinity for substrate and thus inhibiting their activity. ADP, however, functions as an activator of the same enzymes.
ATP acts as an inhibitor, not an activator.
ATP is an allosteric inhibitor. It does not bind to the active site of the enzymes it regulates.
Excess ATP acts as an allosteric inhibitor. It deactivates enzymes by reversibly changing their shape.
Enzyme activity is affected by pH because __________.
- changes in pH can cause loss of cofactors from the enzyme
- the binding of hydrogen ions to the enzyme absorbs energy and thus there may not be enough energy to overcome the activation energy barrier
- low pH will denature all enzymes
- high or low pH may disrupt hydrogen bonding or ionic interactions and thus change the shape of the active site
- most substrates don't function well at high or low pH
high or low pH may disrupt hydrogen bonding or ionic interactions and thus change the shape of the active site
Ex.
Enzyme activity is affected by pH because high or low pH may disrupt hydrogen bonding or ionic interactions and thus change the shape of the active site.
Just as each enzyme has an optimal temperature, it also has a pH at which it is most active. Enzyme activity is affected by pH because high or low pH may disrupt hydrogen bolding or ionic interactions that stabilize the molecule and thus change the shape of the active site. The optimal pH values for most enzymes fall in the range of pH 6–8, but there are exceptions. For example, pepsin, a digestive enzyme in the human stomach, works best at pH 2. Such an acidic environment denatures most enzymes, but pepsin is adapted to maintain its functional three-dimensional structure in the acidic environment of the stomach. In contrast, trypsin, a digestive enzyme residing in the alkaline environment of the human intestine, has an optimal pH of 8 and would be denatured in the stomach.
What would the value of ΔS be for a chemical reaction in which a molecule is broken down into smaller components?
- Negative
- Zero
- Positive
- Neutral
Positive
Ex.
A reaction in which a molecule is broken down into smaller components would have a positive ΔS value.
ΔS is the change in a system’s entropy. When entropy is increased, as in a reaction in which a molecule is broken down, the ΔS value is positive.
ΔS would be zero only in a situation where a chemical reaction does not change the entropy of a system, which is unlikely.
ΔS would be negative in a situation where a chemical reaction reduces the entropy of a system, such as when a molecule is formed from smaller components.
How does ATP drive mechanical work inside a cell?
- By providing free energy to facilitate the formation of polymers from monomers
- By phosphorylating a transport protein
- By removing a phosphate from a transport protein
- By removing free energy from a chemical reaction
- By binding to motor proteins
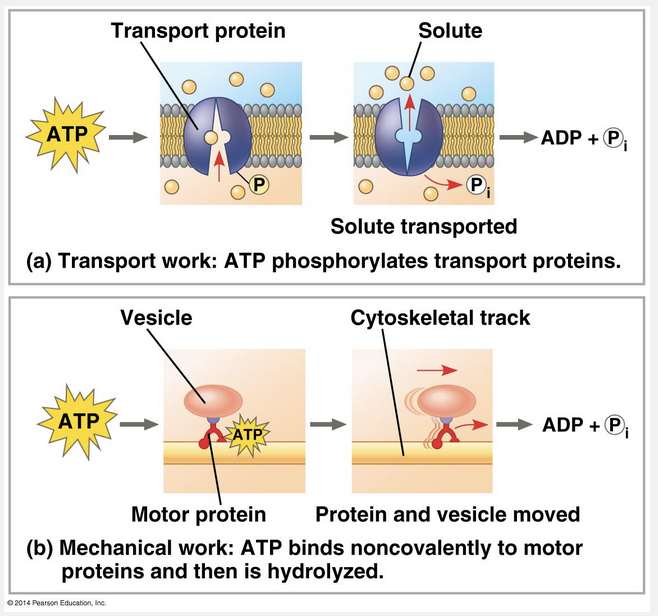
By binding to motor proteins
Ex.
ATP drives mechanical work inside a cell by binding to motor proteins.
Transport and mechanical work in the cell are also nearly always powered by the hydrolysis of ATP. In these cases, ATP hydrolysis leads to changes in a protein’s shape and often its ability to bind to another molecule. Sometimes this occurs via a phosphorylated intermediate. ATP hydrolysis causes changes in the shapes and binding affinities of proteins. This can occur directly, by phosphorylation, for a membrane protein carrying out active transport of a solute or indirectly, via noncovalent binding of ATP and its hydrolytic products, as is the case for motor proteins that move vesicles (and other organelles) along cytoskeletal “tracks” in the cell.
Chemical work is driven by ATP providing free energy to facilitate the formation of polymers from monomers.
ATP adds a phosphate to a transport protein for this kind of work. A phosphate is not removed from the protein.
Mechanical work is driven by ATP binding to motor proteins.
ATP adds free energy to chemical reactions. It does not remove it.
Which of the following reactions would be endergonic?
- ATP → ADP + Pi
- All of the listed responses are correct.
- C6H12O6 + 6 O2 → 6 CO2 + 6 H2O
- HCl → H+ + Cl
- Glucose + fructose → sucrose
Glucose + fructose → sucrose
Ex.
This reaction would be endergonic: Glucose + fructose → sucrose.
An endergonic reaction is one that absorbs free energy from its surroundings. Because this kind of reaction essentially stores free energy in molecules (G increases), ΔG is positive.
Such reactions are nonspontaneous, and the magnitude of ΔG in the equation ΔG = ΔH – TΔS is the quantity of energy required to drive the reaction.
Combining glucose and fructose to produce sucrose is an example of an energy storing endergonic reaction with the product more complex (lower entropy) than the reactants (glucose and fructose).
How does ATP drive transport work inside a cell?
- By providing free energy to facilitate the formation of polymers from monomers
- By phosphorylating a transport protein
- By removing a phosphate from a transport protein
- By removing free energy from a chemical reaction
- By binding to motor proteins
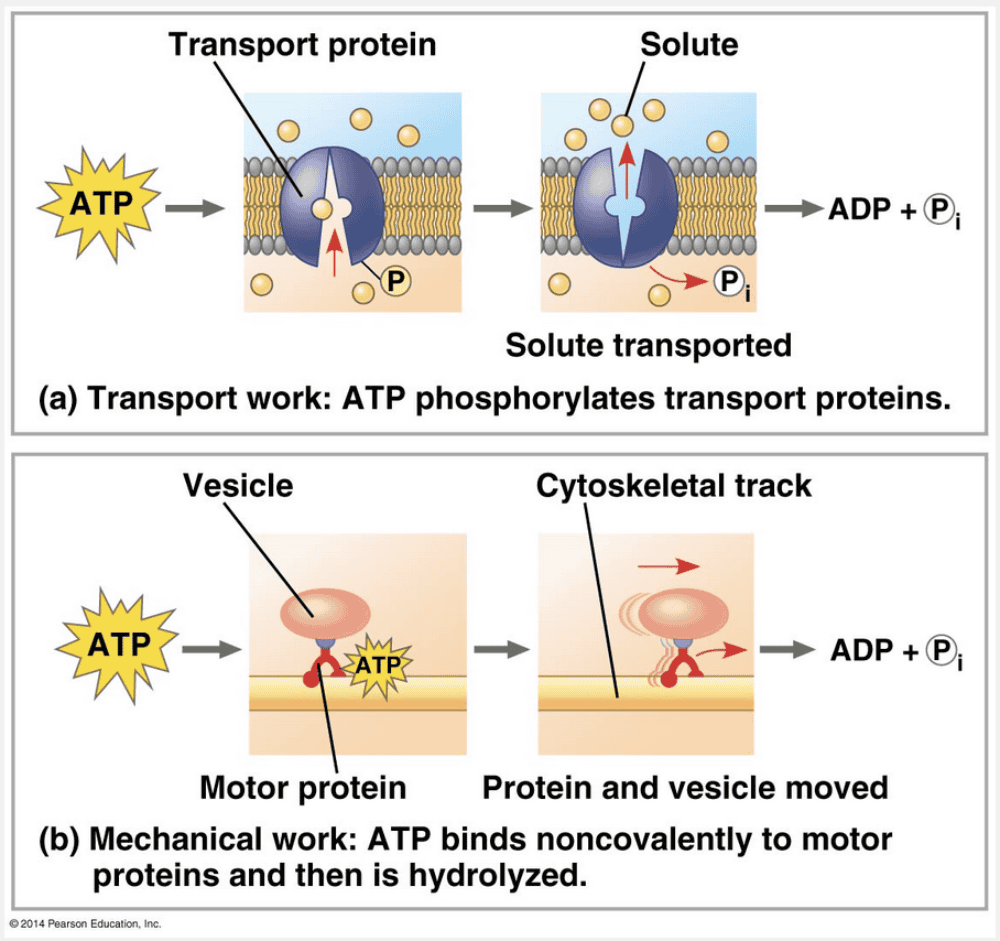
By phosphorylating a transport protein
Ex.
ATP drives transport work inside a cell by phosphorylating a transport protein.
Transport and mechanical work in the cell are also nearly always powered by the hydrolysis of ATP. In these cases, ATP hydrolysis leads to changes in a protein’s shape and often its ability to bind to another molecule. Sometimes this occurs via a phosphorylated intermediate. ATP hydrolysis causes changes in the shapes and binding affinities of proteins. This can occur directly, by phosphorylation, for a membrane protein carrying out active transport of a solute or indirectly, via noncovalent binding of ATP and its hydrolytic products, as is the case for motor proteins that move vesicles (and other organelles) along cytoskeletal “tracks” in the cell.
Mechanical work is driven by ATP binding to motor proteins.
Chemical work is driven by ATP providing free energy to facilitate the formation of polymers from monomers.
ATP adds a phosphate to a transport protein for this kind of work. A phosphate is not removed from the protein.
ATP adds free energy to chemical reactions. It does not remove it.
Which of the following is true regarding metabolic pathways?
- Metabolic pathways consist of a single chemical reaction.
- Metabolic pathways are not important to a cell’s ability to function.
- Metabolic pathways consist of a series of reactions, each catalyzed by a different enzyme.
- Metabolic pathways consist of only anabolic pathways.
- Each reaction in the pathway is catalyzed by the same enzyme.
Metabolic pathways consist of a series of reactions, each catalyzed by a different enzyme.
Ex.
Metabolic pathways consist of a series of reactions, each catalyzed by a different enzyme.
The totality of an organism’s chemical reactions is called metabolism (from the Greek metabole, change). Metabolism is an emergent property of life that arises from orderly interactions between molecules. We can picture a cell’s metabolism as an elaborate road map of the thousands of chemical reactions that occur in a cell, arranged as intersecting metabolic pathways. A metabolic pathway begins with a specific molecule that is then altered in a series of defined steps, resulting in a certain product. Each step of the pathway is catalyzed by a specific enzyme.
Metabolic pathways consist of a series of chemical reactions.
Each reaction in a metabolic pathway is catalyzed by a different enzyme.
Metabolic pathways are very important to how a cell functions, managing the material and energy resources in a cell.
Metabolic pathways can be both anabolic and catabolic.
At low pH, a particular enzyme catalyzes a reaction at a high rate. At neutral pH, the enzyme is completely inactive. What statement best explains the difference in how pH affects the function of this enzyme?
- The enzyme is adapted for low pH but is denatured at neutral pH, leaving it nonfunctional.
- Neutral pH provides the optimal environment in which this enzyme functions.
- Low pH causes the enzyme to denature, and neutral pH causes the enzyme to function normally.
- pH has no effect on enzyme function.
- The enzyme functions best at both low and neutral pH.
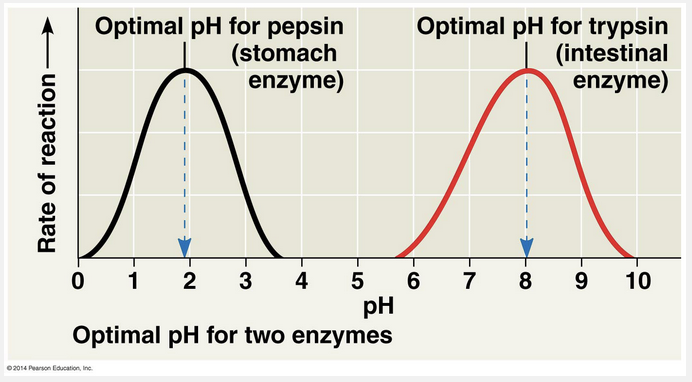
The enzyme is adapted for low pH but is denatured at neutral pH, leaving it nonfunctional.
Ex.
The enzyme is adapted for low pH but is denatured at neutral pH, leaving it nonfunctional.
The activity of an enzyme—how efficiently it functions—is affected by general environmental factors, such as temperature and pH. The three-dimensional structures of proteins are sensitive to their environment. As a consequence, each enzyme works better under some conditions than under other conditions because these optimal conditions favor the most active shape of the enzyme. Temperature and pH are environmental factors important to the activity of an enzyme. Just as each enzyme has an optimal temperature, it also has a pH at which it is most active. The optimal pH values for most enzymes fall in the range of pH 6–8, but there are exceptions. For example, pepsin, a digestive enzyme in the human stomach, works best at pH 2. Such an acidic environment denatures most enzymes, but pepsin has adapted to maintain its functional three-dimensional structure in the acidic environment of the stomach. In contrast, trypsin, a digestive enzyme residing in the alkaline environment of the human intestine, has an optimal pH of 8 and would be denatured in the stomach.
While low pH may cause some enzymes to denature, that is not the case in this situation since the enzyme exhibits a high rate of activity.
Since the enzyme’s function is different in both conditions, pH appears to alter its function.
The enzyme exhibits no activity at neutral pH, indicating that it has likely been denatured.
Due to the difference in function in the different pH environments, pH definitely affects the enzyme’s function.
The process of stabilizing the structure of an enzyme in its active form by the binding of a molecule is an example of __________.
- cooperativity
- competitive inhibition
- feedback inhibition
- noncompetitive inhibition
- allosteric regulation
allosteric regulation
Ex.
The process of stabilizing the structure of an enzyme in its active form by the binding of a molecule is an example of allosteric regulation.
Allosteric regulation involves stabilizing the structure of an enzyme in its active form by the binding of a molecule. Intrinsic to life’s processes is a cell’s ability to tightly regulate its metabolic pathways by controlling when and where its various enzymes are active. It does this either by switching on and off the genes that encode specific enzymes or by regulating the activity of enzymes once they are made. In many cases, the molecules that naturally regulate enzyme activity in a cell behave something like reversible noncompetitive inhibitors: These regulatory molecules change an enzyme’s shape and the functioning of its active site by binding to a site elsewhere on the molecule, via noncovalent interactions.
Allosteric regulation is the term used to describe any case in which a protein’s function at one site is affected by the binding of a regulatory molecule to a separate site. It may result in either inhibition or stimulation of an enzyme’s activity. Most enzymes known to be allosterically regulated are constructed from two or more subunits, each composed of a polypeptide chain with its own active site. The entire complex oscillates between two different shapes, one catalytically active and the other inactive.
In the simplest kind of allosteric regulation, an activating or inhibiting regulatory molecule binds to a regulatory site (sometimes called an allosteric site), often located where subunits join. The binding of an activator to a regulatory site stabilizes the shape that has functional active sites, whereas the binding of an inhibitor stabilizes the inactive form of the enzyme. The subunits of an allosteric enzyme fit together in such a way that a shape change in one subunit is transmitted to all others. Through this interaction of subunits, a single activator or inhibitor molecule that binds to one regulatory site will affect the active sites of all subunits.