Unknown number for hydrate sample?
7 (Trial 1) and 9 (Trial 2)
a) Calculate mass of hydrate for Trial 1. (Show work with short verbal descriptions of numbers. For example, Mass of Crucible with Hydrate - Mass of crucible = 23.40 g - 18.40 g = 5.00 g. If only numbers are shown, the score for this question will be 0.)
b) Calculate mass of the anhydrous salt for Trial 1. (Show work with short verbal descriptions of numbers. If only numbers are shown, the score for this question will be 0.)
c) Calculate the mass of water in the hydrate for Trial 1. (Show work with short verbal descriptions of numbers. If only numbers are shown, the score for this question will be 0.)
mass of hydrate for Trial 1:
Mass of Crucible with Hydrate - Mass of crucible = 27.7437 g - 25.4004 g = 2.3433 g
mass of the anhydrous salt for Trial 1:
Mass of Crucible with anhydrous salt - Mass of crucible = 26.5508 g - 25.4004 g = 1.1504 g
mass of water in the hydrate for Trial 1:
Mass of hydrated salt - Mass of the anhydrous salt = 2.3433 g - 1.1504 g = 1.1929 g
a) Calculate the percentage of water in the hydrate for Trial 1. (Show work with short verbal descriptions of numbers. If only numbers are shown, the score for this question will be 0.)
b) Calculate the average percentage of water in the hydrate for Trials 1 and 2.
percentage of water in the hydrate for Trial 1:
(Molar mass of water / Molar mass of hydrated salt) x 100 = (1.1929 g / 2.3433 g) x 100 = 50.91%
average percentage of water in the hydrate for Trials 1 and 2:
Since me and my partner did our trials on two different unknown substances, Trial 1 for Number 7 and Trial 2 for Number 9, you said that we couldn't average out our percentage of water. However, this is what the average percentage would've looked like if we did so:
(Percentage of water in the hydrate for Trial 1 + Percentage of water in the hydrate for Trial 1) / 2 = (50.91% + 66.24%) / 2 = 58.58%
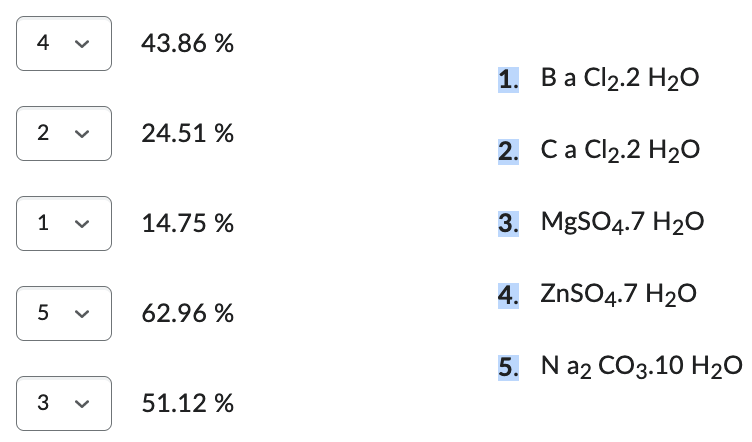
Match the formula of the hydrate with % water in that hydrate.
What is the identity of your unknown?
Magnesium Sulfate Heptahydrate
Your instructor will give you instructions as what to do with this box. It may be used for bonus points, or for taking away points (for example late submissions) or for error analysis if something went wrong during the lab. If no instructions have been provided to you, please leave this box blank.
5% = 5
...