The cation of H3PO4 (acid) (H 3 P O 4 (acid) ) is:
H3 + (OR H3 1+)
WRONG
The cation of CrO is:
Cr2 + (OR Cr2 1+)
WRONG
The anion of FeI2 (OR F e I 2 ) is:
I2 - (OR I2 1 minus)
WRONG
The anion of BeI2 (OR B e I 2 ) is:
I2 - (OR I2 1 minus)
WRONG
The cation of Sn3N2 is:
Sn3 2+
WRONG
The cation of HClO2(acid) (OR HC l O2 (acid)) is:
H+
The anion of NaCl (OR N a Cl) is:
Cl- (OR Cl minus)
The cation of Na2SO3 (N a2 S O3 ) is:
Na2 + OR (N a2 1+)
WRONG
The cation of HI (acid) (OR H I (acid)) is:
H+
The anion of Pb(OH)2 (OR Pb left parenthesis OH right parenthesis 2) is:
(OH)2 - (OR left parenthesis OH right parenthesis 2 1 minus)
WRONG
The anion of PbCl4 is:
Cl4 - (OR Cl4 1 minus)
WRONG
The anion of HClO4 (acid) (OR H C l O4 (acid) ) is:
ClO4 - (OR C l O4 1 minus)
The cation of HF(acid) is:
H+
The anion of CuF (OR C u F) is:
F- (OR F minus)
The anion of HCl (acid) (OR H Cl (acid)) is:
Cl- (OR Cl minus)
The anion for Cu(CN)2 (OR C u left parenthesis CN right parenthesis 2 ) is:
CN2 - (OR CN2 1 minus)
WRONG
The cation of Mg(OH)2 (OR Mg right parenthesis OH left parenthesis 2) is:
Mg2+
The cation of NH4HSO4 (OR N H4 H S O4) is:
NH4 + (OR NH4 1+)
The anion of CrO (OR C r O) is:
O2- (OR O 2 minus)
The anion of Li2O (OR L i 2 O) is:
O2- (OR O 2 minus)
What is the molecular weight of Magnesium fluoride, MgF2 (OR Mg F2). Report your answer to two decimal places.
Do not include units with your answer.
The atomic weight of M g is 24.31 grams/mole
The atomic weight of F is 19.00 grams/mole
62.31
What is the molecular weight of glucose, C6H12O6 (OR C6 H12 O6). Report your answer to two decimal places.
Do not include units with your answer.
The atomic weight of H is 1.01 grams/mole
The atomic weight of O is 16.00 grams/mole
The atomic weight of C is 12.01 grams/mole
180.18
Convert (3.078x10^24) molecules of acetic acid, HC2H3O2 (OR "H" C2 H3 O2) to grams of acetic acid. Avogadro's Number is 6.022x1023.
You must enter your answer in scientific notation. For example, 25 would be expressed as 2.5x10^1.
Note: Your answer is assumed to be reduced to the highest power possible.
3.070 x 102
Convert 151.0 grams of oxygen gas, O2, to liters at STP.
1 mole of gas at STP is 22.414 Liters
105.8
Convert (2.92x10^24) molecules of glucose, C6H12O6 (OR C6 H12 O6) to grams of glucose. Avogadro's number is 6.022x1023.
You must enter your answer in scientific notation. For example, 25 would be expressed as 2.5x10^1.
Note: Your answer is assumed to be reduced to the highest power possible.
8.74 x 102
Convert 97.0 liters of chlorine gas, Cl2, to grams at STP.
1 mole of gas at STP is 22.414 Liters
307
How many molecules are there in 2.5 mole of NaCl (OR "N" a C l)?
Express your answer in scientific notation using E to represent scientific notation. For example, 6.022x10^23 would be represented as 6.022E23.
Round your answers to the proper number of significant figures and do not include units with you answer.
1.5E24
What is the percent of water in N a2C O3 dot
2H2O?
% H2O (MW = 18.02 g H2O) = ______%
25.36
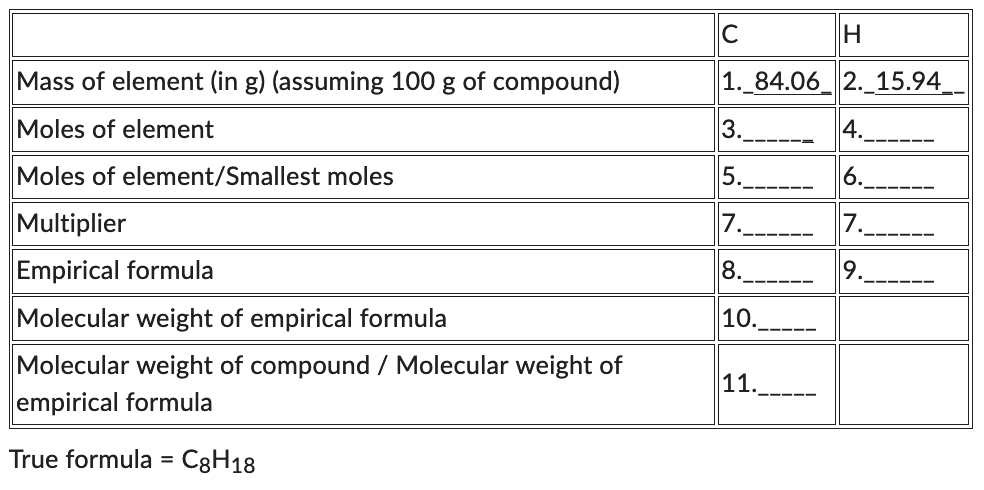
A hydrocarbon has a MW of 114 g/mol and has a composition of carbon 84.06% and hydrogen 15.94%. What is its (a) empirical formula (b) true formula?