The molecule with four fused rings that is found in animal membranes and is the precursor of vertebrate sex hormones is __________.
- cholesterol
- glycerol
- testosterone
- estrogen
- a phospholipid
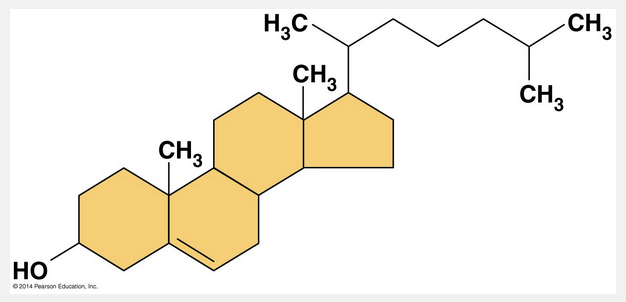
cholesterol
Ex.
The molecule with four fused rings that is found in animal membranes and is the precursor of vertebrate sex hormones is cholesterol.
Steroids are lipids characterized by a carbon skeleton consisting of four fused rings. Different steroids are distinguished by the particular chemical groups attached to this ensemble of rings. Cholesterol, a type of steroid, is a crucial molecule in animals. It is a common component of animal cell membranes and is also the precursor from which other steroids, such as the vertebrate sex hormones, are synthesized. In vertebrates, cholesterol is synthesized in the liver and is also obtained from the diet. A high level of cholesterol in the blood may contribute to atherosclerosis. In fact, both saturated fats and trans fats exert their negative impact on health by affecting cholesterol levels.
“Estrogen” and “testosterone” are incorrect; they are derived from cholesterol and are sex hormones, not components of animal membranes.
“Glycerol” is incorrect because it is a three-carbon straight-chain lipid.
“A phospholipid” is incorrect because even though it is found in animal membranes, it is not a four-ring structure called a steroid.
Carbohydrates are used in our bodies mainly for __________.
- structural molecules, such as hair and fingernails
- lipid storage
- energy storage and release
- membrane construction
- building genetic material
energy storage and release
Ex.
Carbohydrates are used in our bodies mainly for energy storage and release.
Carbohydrates include both sugars and polymers of sugars. The simplest carbohydrates are the monosaccharides, or simple sugars; these are the monomers from which more complex carbohydrates are constructed. Carbohydrates also include macromolecules called polysaccharides, polymers composed of many sugar building blocks. Simple sugar molecules, stored in polysaccharides such as glycogen in animals and starch in plants, are a major energy source for cellular work. Membranes are composed primarily of lipids.
Hair and fingernails are composed primarily of protein.
Genetic material is composed of nucleic acids, and enzymatic proteins catalyze its construction.
Although carbohydrates can be converted to lipids, carbohydrates and lipids are two different categories of the four major kinds of macromolecules found in living systems.
Macromolecules, the molecules of life, include all of the following except __________.
- carbohydrates
- All of the molecules listed are macromolecules.
- proteins
- trace elements
- nucleic acids
trace elements
Ex.
Macromolecules, the molecules of life, include all of the following except trace elements.
Trace elements are elements that are indispensable for life but are required in extremely minute amounts. The macromolecules in three of the four classes of life’s organic compounds—carbohydrates, proteins, and nucleic acids; but not lipids—are chain-like molecules called polymers (from the Greek polys, many, and meros, part). A polymer is a long molecule consisting of many similar or identical building blocks linked by covalent bonds, much as a train consists of a chain of cars. The repeating units that serve as the building blocks of a polymer are smaller molecules called monomers (from the Greek monos, single). Some monomers also have other functions of their own.
The macromolecules in three of the four classes of life’s organic compounds are “carbohydrates,” “proteins,” and “nucleic acids” (the fourth is lipids) and are chain-like molecules called polymers. Since this is an except question, these are incorrect answers.
“All of the molecules listed are macromolecules” is incorrect because trace elements are not macromolecules.
The type of bond that forms to join monomers (such as sugars and amino acids) into polymers (such as starch and proteins) is a(n) __________ bond.
- peptide
- ionic
- hydrogen
- covalent
- van der Waals
covalent
Ex.
The type of bond that forms to join monomers (such as sugars and amino acids) into polymers (such as starch and proteins) is a covalent bond.
Monomers are joined together by a dehydration reaction in which two molecules are covalently bonded to each other through the loss of a water molecule.
A hydrogen bond is a relatively weak bond between polar molecules. An ionic bond cannot form because sugars are not ions.
A peptide bond is a specific type of covalent bond not found in polysaccharides.
Van der Waals interactions do not link monomers together. They play a role in the tertiary structure of proteins.
Nitrogenous bases are classified as either purines or pyrimidines. Examples of purines are __________.
- adenine and uracil
- guanine and cytosine
- adenine and thymine
- adenine and guanine
- thymine and cytosine
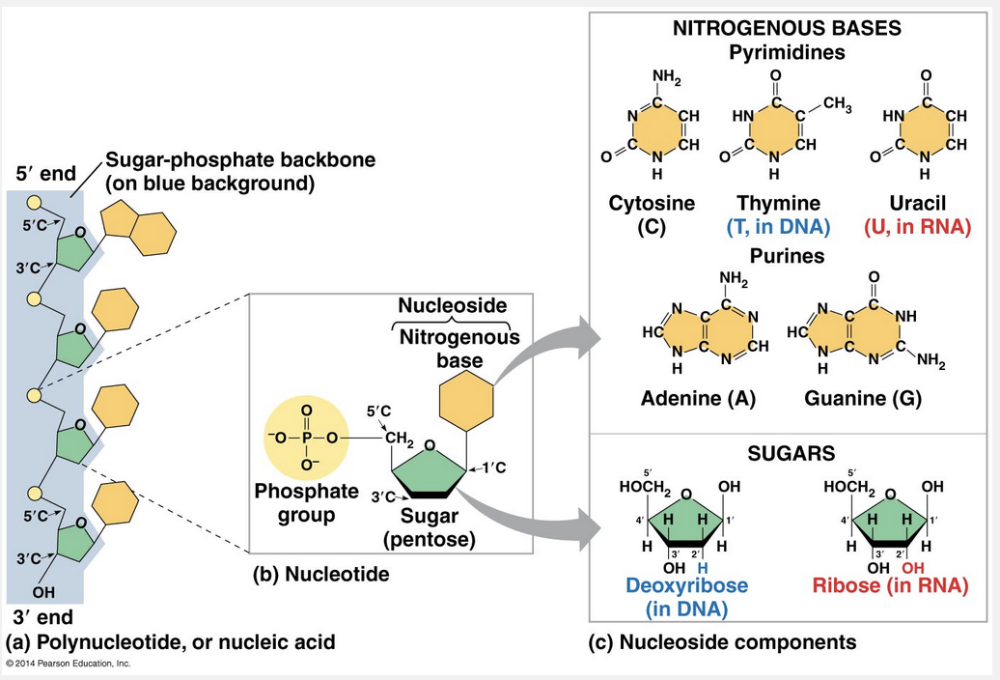
adenine and guanine
Ex.
Nitrogenous bases are classified as either purines or pyrimidines. Examples of purines are adenine and guanine.
To build a nucleotide, let’s first consider the nitrogenous bases. Each nitrogenous base has one or two rings that include nitrogen atoms. (They are called nitrogenous bases because the nitrogen atoms tend to take up H++ from solution, thus acting as bases.) There are two families of nitrogenous bases: pyrimidines and purines. A pyrimidine has one six-membered ring of carbon and nitrogen atoms. The members of the pyrimidine family are cytosine (C), thymine (T), and uracil (U). Purines are larger, with a six-membered ring fused to a five-membered ring. The purines are adenine (A) and guanine (G). The specific pyrimidines and purines differ in the chemical groups attached to the rings. Adenine, guanine, and cytosine are found in both DNA and RNA; thymine is found only in DNA and uracil only in RNA.
“Adenine and thymine” is incorrect because thymine is a pyrimidine and not a purine.
“Thymine and cytosine” is incorrect because are both pyrimidines.
“Guanine and cytosine” is incorrect because cytosine is a pyrimidine and not a purine.
“Adenine and uracil” is incorrect because uracil is a pyrimidine and not a purine.
When comparing saturated and naturally occurring unsaturated fats, the unsaturated fats have __________ and are __________ at room temperature.
- cis double bonds; solids
- single bonds; liquids
- single bonds; solids
- trans double bonds; liquids
- cis double bonds; liquids
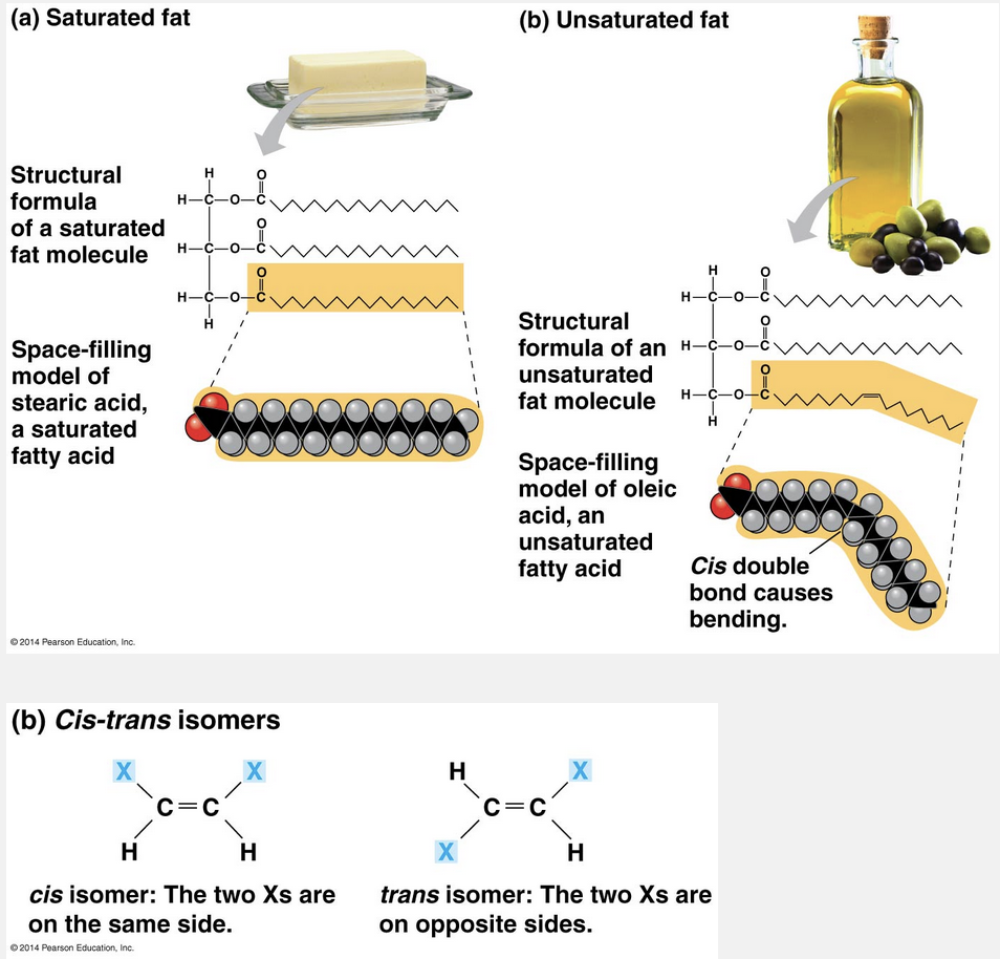
cis double bonds; liquids
Ex.
When comparing saturated and naturally occurring unsaturated fats, the unsaturated fats have cis double bonds and are liquids at room temperature.
The terms saturated fats and unsaturated fats are commonly used in the context of nutrition. These terms refer to the structure of the hydrocarbon chains of the fatty acids. If there are no double bonds between carbon atoms composing a chain, then as many hydrogen atoms as possible are bonded to the carbon skeleton. Such a structure is said to be saturated with hydrogen, and the resulting fatty acid is therefore called a saturated fatty acid. An unsaturated fatty acid has one or more double bonds, with one fewer hydrogen atom on each double-bonded carbon. Nearly all double bonds in naturally occurring fatty acids are cis double bonds, which cause a kink in the hydrocarbon chain wherever they occur.
“Cis double bonds; solids” is incorrect because fats with double bonds are liquids.
“Trans double bonds; liquids” is incorrect because trans double bonds are not naturally occurring fats.
“Single bonds; liquids” is incorrect because saturated fats have single bonds and are solid at room temperature.
“Single bonds; solids” is incorrect because fats that have single bonds and are solid at room temperature are called saturated fats.
Which of the following is a polymer?
- Testosterone, a steroid hormone
- Cellulose, a plant cell wall component
- Triacylglycerol, or fat
- Glucose, an energy-rich molecule
- Fructose, a component of sucrose
Cellulose, a plant cell wall component
Ex.
Cellulose, a plant cell wall component, is a polymer.
The macromolecules in three of the four classes of life’s organic compounds—carbohydrates, proteins, and nucleic acids—are chain-like molecules called polymers. A polymer is a long molecule consisting of many similar or identical building blocks linked by covalent bonds, much as a train consists of a chain of cars. The repeating units that serve as the building blocks of a polymer are smaller molecules called monomers. The polysaccharide cellulose is a major component of plant cell walls. It is a polymer composed of many glucose monomers joined together by glycosidic linkages.
Testosterone is categorized as a lipid, more specifically a steroid. It is not composed of monomer building blocks. The molecules are characterized by a carbon skeleton consisting of four fused rings of carbon atoms.
Glucose is a monosaccharide, a monomer.
Triacylglycerol is a fat composed of three fatty acid molecules joined by ester linkages to one molecule of glycerol.
Fructose is a monosaccharide, a monomer.
The peptide bond is __________.
- a covalent bond joining simple sugars together to form a polypeptide
- a hydrogen bond joining amino acids together to form a polypeptide
- a covalent bond joining nucleotides together to form a nucleic acid
- a hydrogen bond joining nucleotides together to form a nucleic acid
- a covalent bond joining amino acids together to form a polypeptide
a covalent bond joining amino acids together to form a polypeptide
Ex.
The peptide bond is a covalent bond joining amino acids together to form a polypeptide.
When two amino acids are positioned so that the carboxyl group of one is adjacent to the amino group of the other, they can become joined by a dehydration reaction, with the removal of a water molecule. The resulting covalent bond is called a peptide bond. Repeated over and over, this process yields a polypeptide, a polymer of many amino acids linked by peptide bonds.
Peptide bonds are not weak interactions, such as hydrogen bonds.
Peptide bonds do not link simple sugars together.
A peptide bond is not a hydrogen bond, and it does not join nucleotides together.
Nucleotides are joined together by phosphodiester linkages, not peptide bonds.
Sugars are molecules that have __________ C:H:O and are called __________.
- equal amounts of; carbohydrates
- more C and H than O in; proteins
- a 2:1:2 ratio of; carbohydrates
- a 1:2:1 ratio of; carbohydrates
- less C and H than O in; lipids
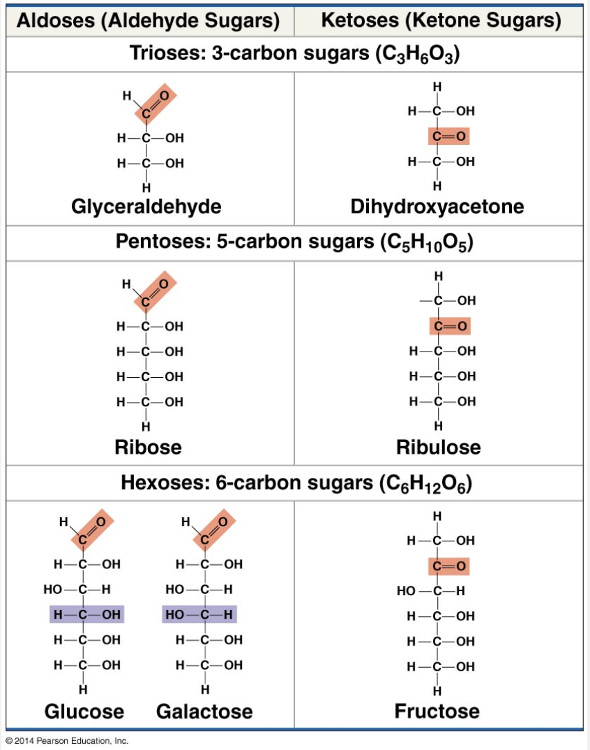
a 1:2:1 ratio of; carbohydrates
Ex.
Sugars are molecules that have a 1:2:1 ratio of C:H:O and are called carbohydrates.
Carbohydrates include sugars and polymers of sugars. The simplest carbohydrates are the monosaccharides, or simple sugars; these are the monomers from which more complex carbohydrates are built. Disaccharides are double sugars consisting of two monosaccharides joined by a covalent bond. Carbohydrate macromolecules are polymers called polysaccharides, which are composed of many sugar building blocks. Monosaccharides (from the Greek monos, single, and sakchar, sugar) generally have molecular formulas that are some multiple of the unit CH2O. Glucose (C6H12O6), the most common monosaccharide, is of central importance in the chemistry of life.
“More C and H than O in; proteins,” “equal amounts of; carbohydrates,” “a 2:1:2 ratio of; carbohydrates,” and “less C and H than O in; lipids” are incorrect because sugars are carbohydrates. For every one carbon molecule (C), there is one molecule of water (H2O). This means that the ratio of C:H:O is 1:2:1.
Protein molecules are polymers (chains) of __________.
- DNA molecules
- amino acid molecules
- fatty acid molecules
- purines and pyrimidines
- sucrose molecules
amino acid molecules
Ex.
Protein molecules are polymers (chains) of amino acid molecules.
Diverse as proteins are, they are all unbranched polymers constructed from the same set of 20 amino acids. Polymers of amino acids are called polypeptides. A protein is a biologically functional molecule that consists of one or more polypeptides, each folded and coiled into a specific three-dimensional structure.
DNA is a polymer of nucleotides.
Fatty acids are a component of triacylglycerols (fats) and phospholipids.
Sucrose is a disaccharide sugar.
Purines and pyrimidines are nitrogenous bases present in nucleotides, the monomers of nucleic acids.
A polysaccharide that is used for storing energy in human muscle and liver cells is __________.
- cellulose
- chitin
- glycogen
- glucose
- starch
glycogen
Ex.
A polysaccharide that is used for storing energy in human muscle and liver cells is glycogen.
Polysaccharides are macromolecules, polymers with a few hundred to a few thousand monosaccharides joined by glycosidic linkages. Some polysaccharides serve as storage material, hydrolyzed as needed to provide sugar for cells. Other polysaccharides serve as building material for structures that protect the cell or the whole organism. Humans and other vertebrates store glucose as a polysaccharide called glycogen in their liver and muscles.
Glucose is a monosaccharide, not a polysaccharide.
Starch is a storage polysaccharide found especially in certain plant tissues.
Chitin is not found in humans. Chitin is a structural polysaccharide found in the exoskeleton of arthropods.
Cellulose is a polysaccharide present in plant cell walls.
The proper three-dimensional shape and folding of a protein is assisted by _________.
- escorts
- a self-assembly process
- complex lipids
- molecules called chaperonins
- complex nucleic acids
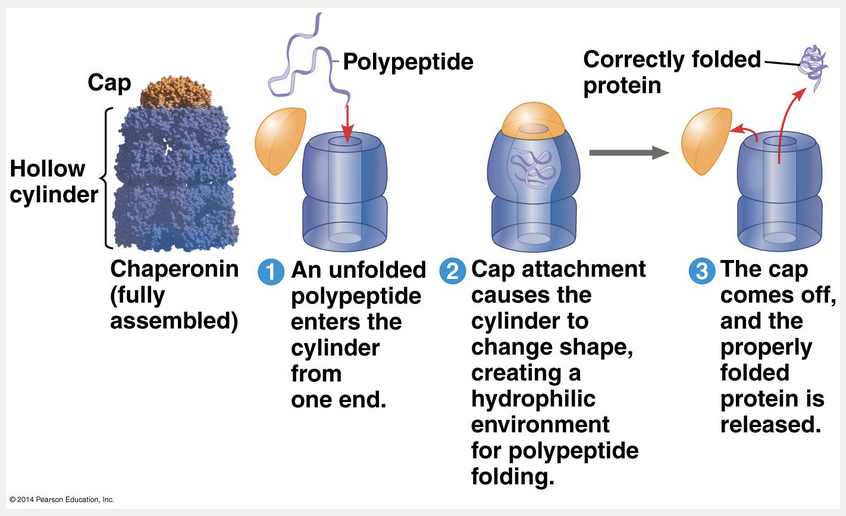
molecules called chaperonins
Ex.
The proper three-dimensional shape and folding of a protein is assisted by molecules called chaperonins.
Biochemists now know the amino acid sequence for more than 24 million proteins, with about 1 million added each month, and the three-dimensional shape for more than 25,000 proteins. Researchers have tried to correlate the primary structure of many proteins with their three-dimensional structure to discover the rules of protein folding. Unfortunately, however, the protein-folding process is not that simple. Most proteins probably go through several intermediate structures on their way to a stable shape, and looking at the mature structure does not reveal the stages of folding required to achieve that form. However, biochemists have developed methods for tracking a protein through such stages. Crucial to the folding process are chaperonins (also called chaperone proteins), protein molecules that assist in the proper folding of other proteins. Chaperonins do not specify the final structure of a polypeptide. Instead, they keep the new polypeptide segregated from disruptive chemical conditions in the cytoplasmic environment while the polypeptide folds spontaneously. The chaperonin shown in the figure below, from the bacterium E. coli, is a giant, multiprotein complex shaped like a hollow cylinder. The cavity provides a shelter for folding polypeptides, and recent research suggests that minute amounts of water are present, thus ensuring a hydrophilic environment that aids the folding process. Molecular systems have also been identified that interact with chaperonins and check whether proper folding has occurred. Such systems either correctly refold the misfolded proteins or mark them for destruction.
“A self-assembly process” is incorrect because chaperonins are crucial to the folding process.
“Escorts” is incorrect because chaperonin is the correct term for molecules that assist in the proper folding of other proteins.
“Complex lipids” and “complex nucleic acids” are incorrect because chaperonins are complex proteins.
The lipids that form the main structural component of cell membranes are __________.
- phospholipids
- proteins
- triacylglycerols
- cholesterol
- carbohydrates
phospholipids
Ex.
The lipids that form the main structural component of cell membranes are phospholipids.
Cells could not exist without another type of lipid—phospholipids. Phospholipids are essential for cells because they make up cell membranes. Their structure provides a classic example of how form fits function at the molecular level. Phospholipids have a hydrophilic head and two hydrophobic tails. This permits the phospholipids to be arranged in a bilayer, or double layer, which forms a boundary between the cell and its external environment.
Triacylglycerols are completely insoluble in aqueous solution and thus have an unsuitable structure for membranes.
Proteins do make up a substantial portion of cell membranes, but proteins are not lipids.
There is some cholesterol in cell membranes, but it is not the main structural component.
Carbohydrates are not lipids.
__________ is always involved in hydrolysis reactions.
- ATP
- H+ and OH–
- Synthesis
- Water
- None of the listed responses is correct.
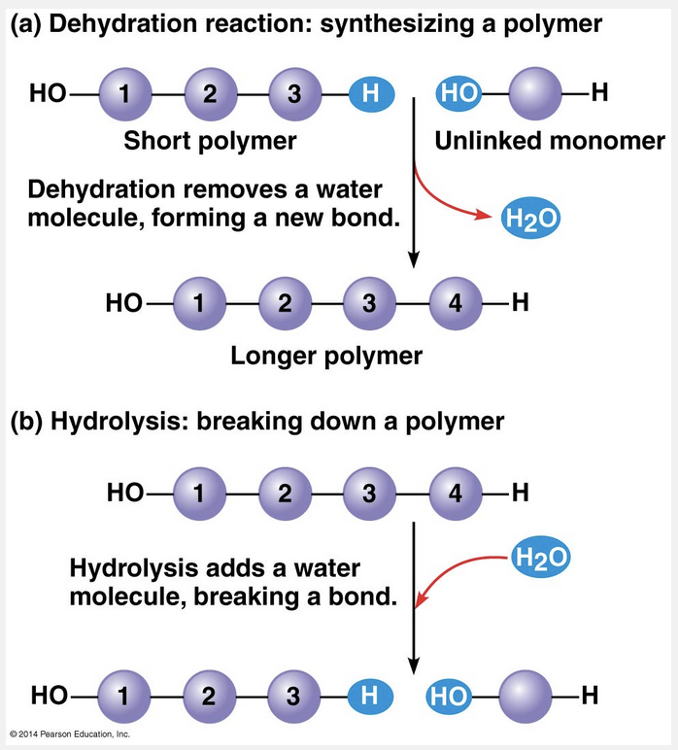
Water
Ex.
Water is always involved in hydrolysis reactions.
Polymers are disassembled to monomers by hydrolysis, a process that is essentially the reverse of the dehydration reaction. Hydrolysis means “water breakage” (from the Greek hydro, water, and lysis, break). The bond between monomers is broken by the addition of a water molecule, with a hydrogen group from water attaching to one monomer and the hydroxyl group attaching to the other monomer.
An example of hydrolysis within our bodies is the process of digestion. The bulk of the organic material in our food is in the form of polymers that are much too large to enter our cells. Within the digestive tract, various enzymes attack the polymers, speeding up hydrolysis. Released monomers are then absorbed into the bloodstream for distribution to all body cells. Those cells can then use dehydration reactions to assemble the monomers into new, different polymers that can perform the specific functions required by each cell.
“H+ and OH–” is incorrect because water is hydrolyzed to produce H+ and O so that when the bond between monomers is broken by the addition of a water molecule, a hydrogen group from water attaches to one monomer and the hydroxyl group attaches to the other monomer.
“ATP” is incorrect because ATP is cellular energy and is not needed for every hydrolysis reaction.
“Synthesis” is incorrect because hydrolysis is not a synthesis reaction but, rather, the process of digestion.
The sex hormones estrogen, progesterone, and testosterone belong to which class of molecules?
- Proteins
- Lipids
- Amino Acids
- Nucleic Acids
- Carbohydrates
Lipids
Ex.
The sex hormones estrogen, progesterone, and testosterone belong to lipids.
Steroids, such as estrogen, progesterone, and testosterone, are lipids based on their insolubility in water. The molecules are characterized by a carbon skeleton consisting of four fused rings of carbon atoms. Different steroids, such as cholesterol and the vertebrate sex hormones, are distinguished by the particular chemical groups attached to this ensemble of rings.
Sex hormones are not proteins, amino acids, carbohydrates, or nucleic acids.
The secondary structure of a peptide backbone is stabilized by __________ forming either a(n) __________ or a(n) __________.
- covalent bonds; α helix; β pleated sheet
- covalent bonds; β helix; α pleated sheet
- ionic bonds; α helix; β pleated sheet
- hydrogen bonds; β helix; α pleated sheet
- hydrogen bonds; α helix; β pleated sheet
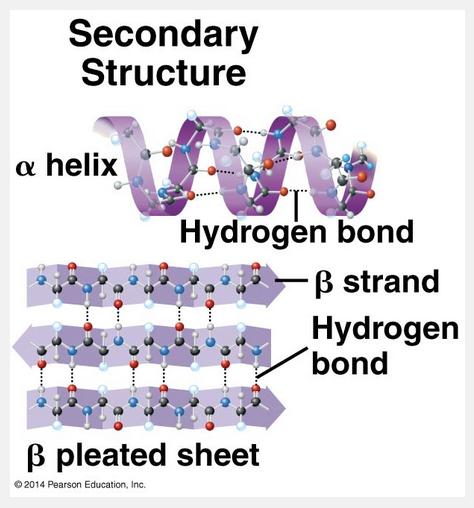
hydrogen bonds; α helix; β pleated sheet
Ex.
The secondary structure of a peptide backbone is stabilized by hydrogen bonds forming either an α helix or a β pleated sheet.
Most proteins have segments of their polypeptide chains repeatedly coiled or folded in patterns that contribute to the protein’s overall shape. These coils and folds, collectively referred to as secondary structure, are the result of hydrogen bonds between the repeating constituents of the polypeptide backbone (not the amino acid side chains). Within the backbone, the oxygen atoms have a partial negative charge, and the hydrogen atoms attached to the nitrogen atoms have a partial positive charge; therefore, hydrogen bonds can form between these atoms. Individually, these hydrogen bonds are weak, but because there are so many of them over a relatively long region of the polypeptide chain, they can support a particular shape for that part of the protein. One such secondary structure is the α helix, a delicate coil held together by hydrogen bonding between every fourth amino acid, as shown below. Although each transthyretin polypeptide has only one α helix, other globular proteins have multiple stretches of α helix separated by nonhelical regions. Some fibrous proteins, such as α-keratin, the structural protein of hair, have the α helix structure over most of their length. The other main type of secondary structure is the β pleated sheet. As shown below, in this structure two or more segments of the polypeptide chain lying side by side (called β strands) are connected by hydrogen bonds between parts of the two parallel segments of the polypeptide backbone.
“Hydrogen bonds; β helix; α pleated sheet” is incorrect because the helix is denoted by an α and the sheet is denoted by a β.
“Covalent bonds; α helix; β pleated sheet” is incorrect because the peptide bond is stabilized by hydrogen bonds.
“Covalent bonds; β helix; α pleated sheet” is incorrect because the peptide bond is stabilized by hydrogen bonds; and the helix is denoted by an α and the sheet is denoted by a β.
“Ionic bonds; α helix; β pleated sheet” is incorrect because hydrogen bonds stabilize the α helix or β pleated sheet. Ionic bond interactions occur because of R group interactions and give the molecule its tertiary structure.
Sickle-cell anemia is a disease that is caused by __________ in the __________ of the protein.
- a single amino acid change; secondary structure
- an amino acid change in the α subunit; primary structure
- abnormal hemoglobin; quaternary structure
- a single amino acid change; primary structure
- multiple amino acid changes; primary structure
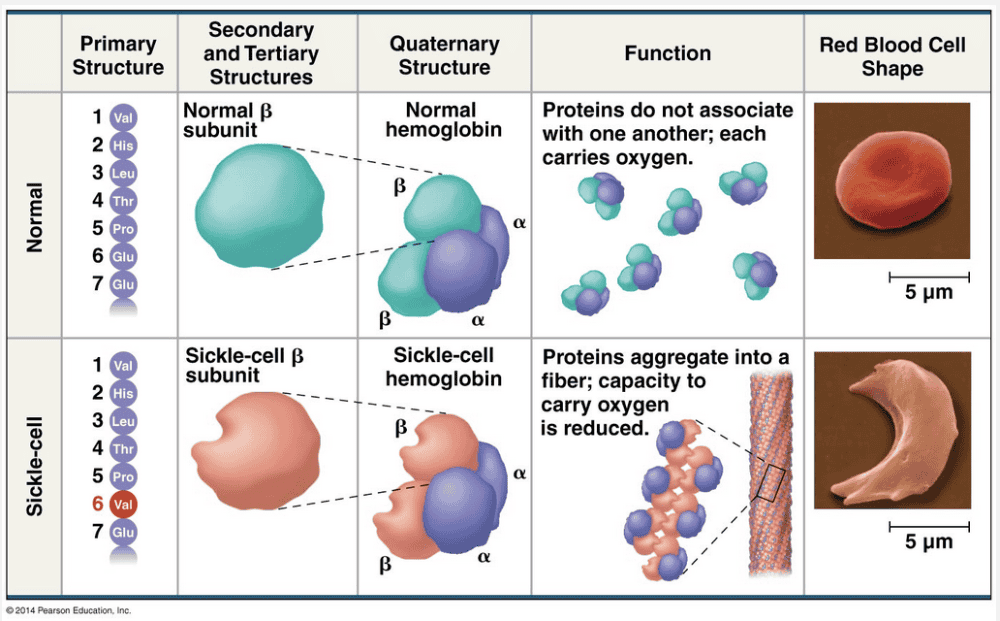
a single amino acid change; primary structure
Ex.
Sickle-cell anemia is a disease that is caused by a single amino acid change in the primary structure of the protein.
Even a slight change in primary structure can affect a protein’s shape and ability to function. For instance, sickle-cell disease, an inherited blood disorder, is caused by the substitution of one amino acid (valine) for the normal one (glutamic acid) at a particular position in the primary structure of hemoglobin, the protein that carries oxygen in red blood cells. Normal red blood cells are disk-shaped, but in sickle-cell disease, the abnormal hemoglobin molecules tend to aggregate into chains, deforming some of the cells into a sickle shape. A person with the disease has periodic “sickle-cell crises” when the angular cells clog tiny blood vessels, impeding blood flow. The toll taken on such patients is a dramatic example of how a simple change in protein structure can have devastating effects on protein function.
“Multiple amino acid changes; primary structure” is incorrect because in sickle-cell disease, a single amino acid substitution from glutamic acid to valine alters the shape of the β subunit of hemoglobin.
“A single amino acid change; secondary structure” is incorrect because an amino acid change is a change in the primary structure of a protein.
“An amino acid change in the α subunit; primary structure” is incorrect because the amino acid change occurs in the α β subunit.
“Abnormal hemoglobin; quaternary structure” is incorrect because the substitution from glutamic acid to valine is a change in the primary structure of the protein.
One characteristic shared by sucrose, lactose, and maltose is that __________.
- they all contain fructose
- they are all monosaccharides
- they are all indigestible by humans
- they are all polysaccharides
- they are all disaccharides
they are all disaccharides
Ex.
One characteristic shared by sucrose, lactose, and maltose is that they are all disaccharides.
Carbohydrates include both sugars and polymers of sugars. The simplest carbohydrates are the monosaccharides, or simple sugars; these are the monomers from which more complex carbohydrates are constructed. Carbohydrates also include macromolecules called polysaccharides, polymers composed of many sugar building blocks. Disaccharides, such as sucrose, maltose, and lactose, are double sugars, consisting of two monosaccharides joined by a glycosidic linkage.
The molecules listed are not polysaccharides. A polysaccharide is composed of many monosaccharide building blocks.
The molecules listed are not monosaccharides. A monosaccharide is a simple sugar containing three to seven carbons.
Fructose is a six-carbon monosaccharide sugar and is not a component of all three of these molecules.
All three of these molecules are digestible by humans.
The sequence of amino acids in a protein is called the __________ structure of the protein.
- primary
- secondary
- quaternary
- tertiary
- None of the listed responses is correct.
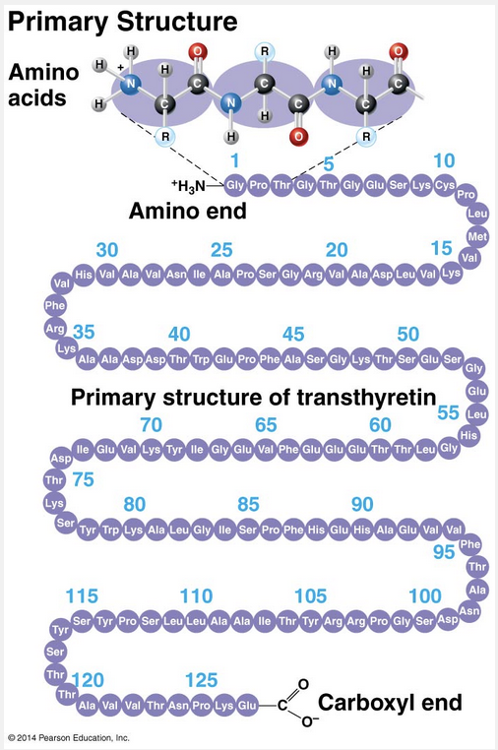
primary
Ex.
The sequence of amino acids in a protein is called the primary structure of the protein.
As an example, let’s consider transthyretin, a globular blood protein that transports vitamin A and one of the thyroid hormones throughout the body. Transthyretin is made up of four identical polypeptide chains, each composed of 127 amino acids. Shown here is one of these chains unraveled to provide a closer look at its primary structure. Each of the 127 positions along the chain is occupied by one of the 20 amino acids, indicated here by its three-letter abbreviation. The primary structure is like the order of letters in a very long word. If left to chance, there would be 20,127 different ways of making a polypeptide chain that is 127 amino acids long. However, the precise primary structure of a protein is determined not by the random linking of amino acids, but by inherited genetic information. The primary structure in turn dictates secondary and tertiary structures, by means of the chemical nature of the backbone and the side chains (R groups) of the amino acids along the polypeptide.
“Secondary” is incorrect because secondary structure is the result of hydrogen bond formation.
“Tertiary” is incorrect because tertiary structure is the result of R group interactions.
“Quaternary” is incorrect because quaternary structure is the result of two or more peptides interacting with each other.
Which is the term for compounds that do not mix with water?
- Hydrogen-bonded
- Hydrophilic
- Phospholipids
- Proteins
- Hydrophobic
Hydrophobic
Ex.
Compounds that do not mix with water are hydrophobic.
The compounds called lipids are grouped together because they share one important trait: They mix poorly, if at all, with water. Hydrophobic compounds are those that are insoluble in water. This hydrophobic behavior of lipids is based on their molecular structure.
Phospholipids are partially soluble in an aqueous medium because of their hydrophilic "heads."
Hydrophilic compounds are those that are soluble in water.
Proteins may be soluble or insoluble in water depending on the number and types of their R (side) groups.
Although hydrogen-bonded compounds are likely to be water-soluble, this is not the term that means "do not mix with water."
Which type of protein shields a newly forming protein from cytoplasmic influences while it is folding into its functional form?
- Receptor proteins
- Fibrous proteins
- Enzymes
- Antibodies
- Chaperonins
Chaperonins
Ex.
Chaperonins, a type of protein shields a newly forming protein from cytoplasmic influences while it is folding into its functional form.
A polypeptide chain of a given amino acid sequence can spontaneously arrange itself into a three-dimensional shape determined and maintained by the interactions responsible for secondary and tertiary structure. This folding normally occurs as the protein is being synthesized in the crowded environment within a cell, aided by other proteins. Crucial to the folding process are chaperonins (also called chaperone proteins), protein molecules that assist in the proper folding of other proteins. Chaperonins shield proteins from "bad influences" (interactions with other molecules in the cytoplasm) while they are folding into their functional forms.
Antibodies have a specific form that allows them to bind to foreign materials in the body.
Enzymes are proteins that act as biological catalysts.
Fibrous proteins usually function as structural proteins.
Receptor proteins bind signaling molecules such as endorphins that may change the behavior of a cell.
In a dehydration synthesis reaction, __________ is always formed as a by-product of the reaction.
- ATP
- water
- H+ and OH–
- heat
- None of the listed responses is correct.

water
Ex.
In a dehydration synthesis reaction, water is always formed as a by-product of the reaction.
Although each class of polymer is made up of a different type of monomer, the chemical mechanisms by which cells make and break down polymers are basically the same in all cases. In cells, these processes are facilitated by enzymes, specialized macromolecules that speed up chemical reactions. Monomers are connected by a reaction in which two molecules are covalently bonded to each other, with the loss of a water molecule; this is known as a dehydration reaction. When a bond forms between two monomers, each monomer contributes part of the water molecule that is released during the reaction: One monomer provides a hydroxyl group (–OH), while the other provides a hydrogen group (–H). This reaction is repeated as monomers are added to the chain one by one, making a polymer.
“H+ and OH–” is incorrect because during a dehydration reaction, one monomer provides a hydroxyl group (–OH), while the other provides a hydrogen group (–H) for the water molecule that is released during the reaction.
“ATP” is incorrect because cellular energy is not produced in this type of reaction.
“Heat” is incorrect because heat is not released from a dehydration reaction.
At a conference, the speaker's grand finale was sautéing mealworms (insect larvae) in butter and serving them to the audience. They were crunchy (like popcorn hulls) because their exoskeletons contain the polysaccharide __________.
- collagen
- chitin
- glycogen
- linoleic acid
- cellulose
chitin
Ex.
At a conference, the speaker's grand finale was sautéing mealworms (insect larvae) in butter and serving them to the audience. They were crunchy (like popcorn hulls) because their exoskeletons contain the polysaccharide chitin.
Polysaccharides are macromolecules, polymers with a few hundred to a few thousand monosaccharides joined by glycosidic linkages. Some polysaccharides serve as storage material, hydrolyzed as needed to provide sugar for cells. Other polysaccharides serve as building material for structures that protect the cell or the whole organism. Chitin is the structural polysaccharide found in arthropod exoskeletons.
Collagen is a structural protein found in tendons and ligaments (among other places).
Cellulose is a structural polysaccharide found in plant cell walls.
For humans, linoleic acid is an essential fatty acid. In animals, glycogen is a glucose storage form of polysaccharide.
The molecule with four fused rings that is found in animal membranes and is the precursor of vertebrate sex hormones is __________.
- a phospholipid
- glycerol
- cholesterol
- testosterone
- estrogen

cholesterol
Ex.
The molecule with four fused rings that is found in animal membranes and is the precursor of vertebrate sex hormones is cholesterol.
Steroids are lipids characterized by a carbon skeleton consisting of four fused rings. Different steroids are distinguished by the particular chemical groups attached to this ensemble of rings. Cholesterol, a type of steroid, is a crucial molecule in animals. It is a common component of animal cell membranes and is also the precursor from which other steroids, such as the vertebrate sex hormones, are synthesized. In vertebrates, cholesterol is synthesized in the liver and is also obtained from the diet. A high level of cholesterol in the blood may contribute to atherosclerosis. In fact, both saturated fats and trans fats exert their negative impact on health by affecting cholesterol levels.
“Estrogen” and “testosterone” are incorrect; they are derived from cholesterol and are sex hormones, not components of animal membranes.
“Glycerol” is incorrect because it is a three-carbon straight-chain lipid.
“A phospholipid” is incorrect because even though it is found in animal membranes, it is not a four-ring structure called a steroid.
In living organisms, DNA exists as a __________ with the strand(s) __________.
- single strand; coiled into a helix
- triple helix; as a twisted coil
- double helix; running parallel
- double helix; running antiparallel
- triple helix; running parallel
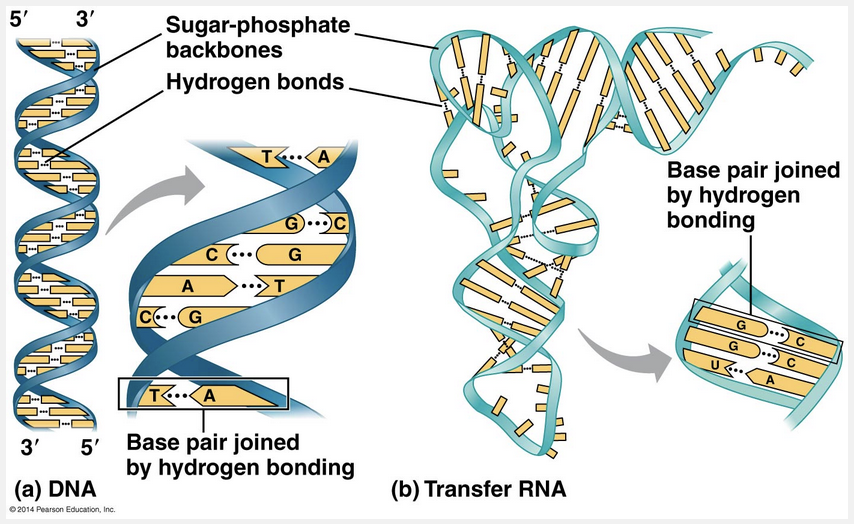
double helix; running antiparallel
Ex.
In living organisms, DNA exists as a double helix with the strands running antiparallel.
DNA molecules have two polynucleotides, or “strands,” that wind around an imaginary axis, forming a double helix. The two sugar-phosphate backbones run in opposite 5′ → 3′ directions from each other; this arrangement is referred to as antiparallel and is somewhat like a divided highway. The sugar-phosphate backbones are on the outside of the helix, and the nitrogenous bases are paired on the interior of the helix. The two strands are held together by hydrogen bonds between the paired bases. Most DNA molecules are very long, with thousands or even millions of base pairs. For example, the one long DNA double helix in a eukaryotic chromosome includes many genes, each one a particular segment of the molecule.
“Double helix; running parallel” is incorrect because the DNA strands run in opposite directions, or are antiparallel.
“Triple helix; as a twisted coil” is incorrect because DNA is a double helix with the strands running antiparallel to each other.
“Single strand; coiled into a helix” is incorrect because DNA is a double helix with the strands running antiparallel to each other.
“Triple helix; running parallel” is incorrect because DNA is a double helix with the strands running antiparallel to each other.
The subunits (monomers) in cellulose are linked together by __________.
- peptide bonds
- phosphodiester linkages
- ester linkages
- glycosidic linkages
- ionic bonds
glycosidic linkages
Ex.
The subunits (monomers) in cellulose are linked together by glycosidic linkages.
Polysaccharides are macromolecules, polymers with a few hundred to a few thousand monosaccharides joined by covalent bonds. The glucose monomers of cellulose are linked together by a specific type of covalent bond known as a glycosidic linkage.
Carbohydrate monomers are not ions linked by ionic bonds.
Peptide bonds link amino acids.
Nucleotides are joined together by phosphodiester linkages and fatty acids are joined to glycerol by ester linkages.
Generally, animals cannot digest (hydrolyze) the glycosidic linkages between the glucose molecules in cellulose. How then do cows get enough nutrients from eating grass?
- The flat teeth and strong stomach of herbivores break the cellulose fibers so that the cows get enough nutrition from the cell contents.
- Cows and other herbivores are exceptions and make some cellulose-digesting enzymes.
- All of the listed responses are correct.
- Microorganisms in their digestive tracts hydrolyze the cellulose to individual glucose units.
- They have to eat a lot of it.
Microorganisms in their digestive tracts hydrolyze the cellulose to individual glucose units.
Ex.
Generally, animals cannot digest (hydrolyze) the glycosidic linkages between the glucose molecules in cellulose. Cows get enough nutrients from eating grass because microorganisms in their digestive tracts hydrolyze the cellulose to individual glucose units.
The polysaccharide called cellulose is a major component of the tough walls that enclose plant cells. Like starch, cellulose is a polymer of glucose, but the glycosidic linkages in these two polymers differ. In starch, all the glucose monomers are in the α configuration. In contrast, the glucose monomers of cellulose are all in the β configuration, making every glucose monomer “upside down” with respect to its neighbors. Enzymes that digest starch by hydrolyzing its α linkages are unable to hydrolyze the β linkages of cellulose because of the distinctly different shapes of these two molecules. In fact, few organisms possess enzymes that can digest cellulose. Cows have digestive chambers populated by microorganisms that can produce certain hydrolytic enzymes that cows cannot. The enzymes hydrolyze (digest) the cellulose polymer into glucose monomers.
Eating more grass would not make it more digestible.
Cows cannot digest cellulose without the help of their microorganismal flora, because they cannot produce an enzyme to hydrolyze the specific β glycosidic linkages present in the cellulose polymer.
The teeth and stomach do not impact digestion of grass in cows.
The components of nucleic acids are __________.
- a nitrogenous base, a pentose sugar, and a phosphate
- a nitrogenous base and a hexose sugar
- a nitrogenous base, a hexose sugar, and a phosphate
- a nitrogenous base and a phosphate
- a nitrogenous base and a pentose sugar
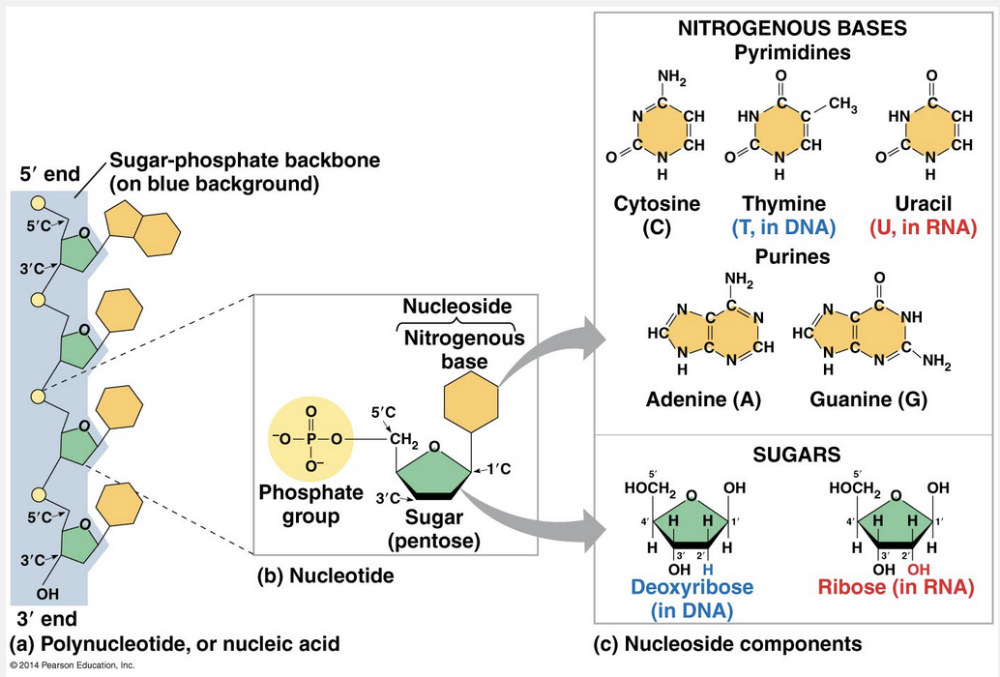
a nitrogenous base, a pentose sugar, and a phosphate
Ex.
The components of nucleic acids are a nitrogenous base, a pentose sugar, and a phosphate.
Nucleic acids are macromolecules that exist as polymers called polynucleotides. As indicated by the name, each polynucleotide consists of monomers called nucleotides. A nucleotide, in general, is composed of three parts: a five-carbon sugar (a pentose), a nitrogen-containing (nitrogenous) base, and one or more phosphate groups. In a polynucleotide, each monomer has only one phosphate group. The portion of a nucleotide without any phosphate group is called a nucleoside.
“A nitrogenous base, a hexose sugar, and a phosphate” is incorrect because the sugar in nucleic acids is a five-carbon sugar called a pentose sugar.
“A nitrogenous base and a pentose sugar” is incorrect because since this lacks a phosphate group, it is called a nucleoside.
“A nitrogenous base and a hexose sugar” is incorrect because hexose sugars are not components of nucleic acids.
“A nitrogenous base and a phosphate” is incorrect because nucleic acid always have a pentose sugar.
A shortage of phosphorus in the soil would make it especially difficult for a plant to manufacture __________.
- fatty acids
- sucrose
- proteins
- DNA
- cellulose
DNA
Ex.
A shortage of phosphorus in the soil would make it especially difficult for a plant to manufacture DNA.
Nucleic acids are macromolecules that exist as polymers called polynucleotides. DNA (deoxyribonucleic acid) is an example of a nucleic acid. As indicated by the name, each polynucleotide consists of monomers called nucleotides. A nucleotide, in general, is composed of three parts: a nitrogen-containing (nitrogenous) base, a five-carbon sugar (a pentose), and one or more phosphate groups. In a polynucleotide, each monomer has only one phosphate group. The backbone of a nucleic acid consists of alternating sugar and phosphate groups.
Proteins are made up of amino acids, and none of the amino acids used by living organisms contains phosphorus.
Cellulose is a carbohydrate. It consists of carbon, oxygen, and hydrogen.
Fatty acids have long carbon skeletons with a carbonyl group at one end. They do not contain phosphorus.
Sucrose is a disaccharide sugar; it does not contain phosphorus.
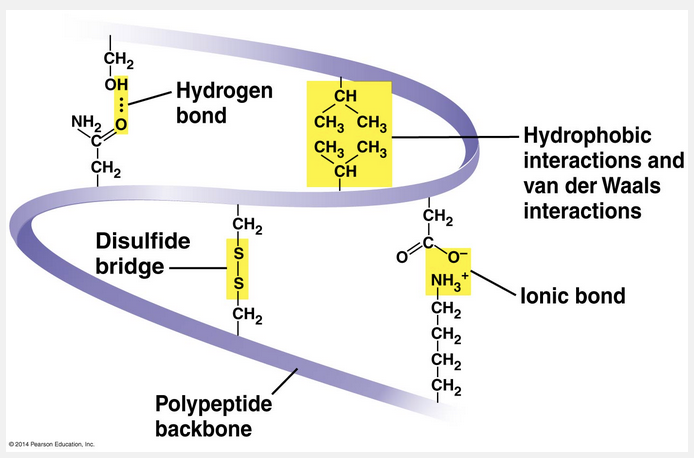
The tertiary structure of a protein includes all of the following interactions except _________ bonds.
- disulfide bridge
- peptide
- ionic
- hydrophobic
- hydrophilic
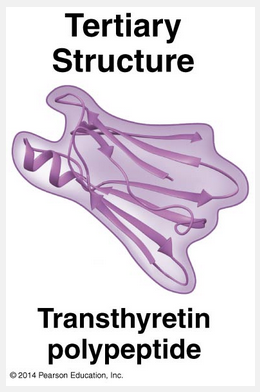
peptide
Ex.
The tertiary structure of a protein includes all of the following interactions except peptide bonds.
Superimposed on the patterns of secondary structure is a protein’s tertiary structure, shown below in the ribbon model of the transthyretin polypeptide. While secondary structure involves interactions between backbone constituents, tertiary structure is the overall shape of a polypeptide resulting from interactions between the side chains (R groups) of the various amino acids.
One type of interaction that contributes to tertiary structure is called—somewhat misleadingly—a hydrophobic interaction. As a polypeptide folds into its functional shape, amino acids with hydrophobic (nonpolar) side chains usually end up in clusters at the core of the protein, out of contact with water. Thus, a “hydrophobic interaction” is actually caused by the exclusion of nonpolar substances by water molecules. Once nonpolar amino acid side chains are close together, van der Waals interactions help hold them together.
Meanwhile, hydrogen bonds between polar side chains and ionic bonds between positively and negatively charged side chains also help stabilize tertiary structure. These are all weak interactions in the aqueous cellular environment, but their cumulative effect helps give the protein a unique shape. Covalent bonds called disulfide bridges may further reinforce the shape of a protein. Disulfide bridges form where two cysteine monomers, which have sulfhydryl groups (–SH) on their side chains, are brought close together by the folding of the protein. The sulfur of one cysteine bonds to the sulfur of the second, and the disulfide bridge (–S–S–) rivets parts of the protein together.
All of these different kinds of interactions can contribute to the tertiary structure of a protein, as shown here in a small part of a hypothetical protein: (PICTURE WITH QUESTION ON THE LEFT)
“Hydrophobic,” “hydrophilic,” “ionic,” and “disulfide bridge” are incorrect because they are interactions that contribute to the tertiary structure of a protein. Since this is an except question, the correct answer is peptide bonds.
Sugars have a(n) __________ group that interacts with a _________ group that forms ring structures when the dry molecule is placed in water.
- carboxyl (-COOH); hydroxyl (-OH)
- carbonyl (-C=O); hydroxyl (-OH)
- hydroxyl (-OH); hydroxyl (-OH)
- amino (-NH2); hydroxyl (-OH)
- carbonyl (-C=O); carboxyl (-COOH)
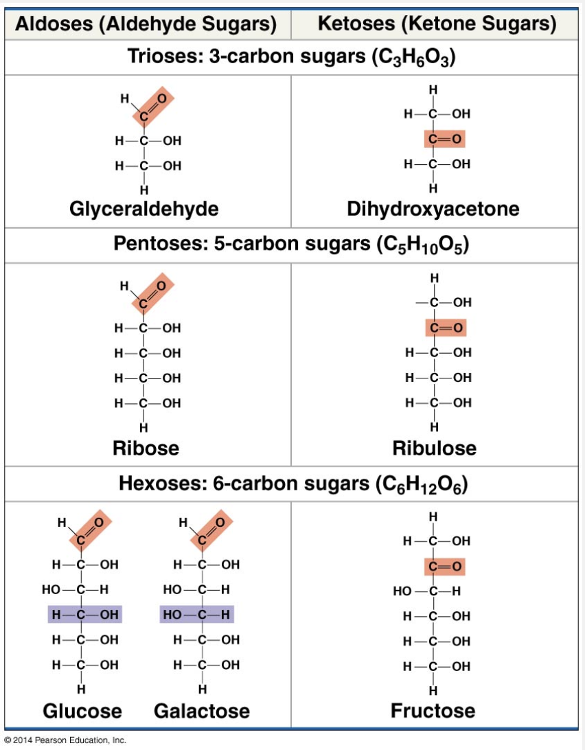
carbonyl (-C=O); hydroxyl (-OH)
Ex.
Sugars have a carbonyl (-C=O) group that interacts with a hydroxyl (-OH) group that forms ring structures when the dry molecule is placed in water.
Monosaccharides (from the Greek monos, single, and sakchar, sugar) generally have molecular formulas that are some multiple of the unit CH2O. Glucose (C6H12O6), the most common monosaccharide, is of central importance in the chemistry of life. In the structure of glucose, we can see the trademarks of a sugar: The molecule has a carbonyl group (CO) and multiple hydroxyl groups (–OH). Depending on the location of the carbonyl group, a sugar is either an aldose (aldehyde sugar) or a ketose (ketone sugar). Glucose, for example, is an aldose; fructose, an isomer of glucose, is a ketose. (Most names for sugars end in the suffix –ose.) Another criterion for classifying sugars is the size of the carbon skeleton, which ranges from three to seven carbon molecules long. Glucose, fructose, and other sugars that have six carbon molecules are called hexoses. Trioses (three-carbon sugars) and pentoses (five-carbon sugars) are also common.
“Carboxyl (-COOH); hydroxyl (-OH)” is incorrect because a carboxyl group is an acid group and is not found in carbohydrates.
“Carbonyl (-C=O); carboxyl (-COOH)” is incorrect because a carboxyl group is an acid group and is not found in carbohydrates.
“Hydroxyl (-OH); hydroxyl (-OH)” is incorrect because although sugars have multiple hydroxyl groups, they also have a carbonyl group.
“Amino (-NH2); hydroxyl (-OH)” is incorrect because sugars have only C, H, and O atoms.