Screen Reader Note. Absolute value of (Experimental Density minus known Density). This divided by Known Density. Everything is multiplied by 100 percent. End of note.
The specific gravity of aluminum is 2.70. A student measured the specific gravity of an aluminum bar and found it to be 3.15. Express the units as % (in the units box). Round to the nearest whole number.
Percent Error = (3.15 - 2.70) / 2.70 x 100% = 16.6%
A flask is found to have a mass of 119.45 grams. A liquid is added to a buret, and the initial measurement is found to be 0.55 mL. After liquid has been added to the flask from the buret, the buret reads 12.95 mL. The flask is weighed again, and is found to have a mass of 146.55 grams. What is the density of the liquid in g/mL?
Mass of water = 146.55 g - 119.45 g = 27.100 g
Volume of water = 12.95 mL - 0.55 mL = 12.4 mL
Density of liquid = 27.100 g / 12.4 mL = 2.2 g/mL
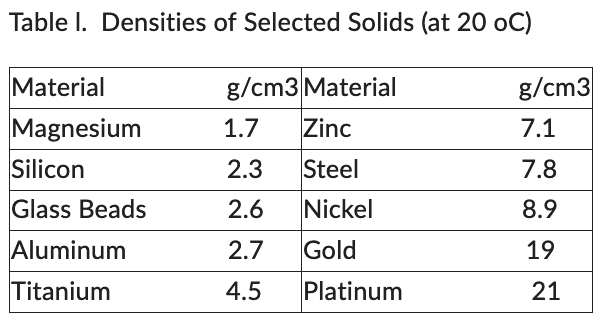
Pellets of a certain substance are found to weigh 17.50 g. A volume of 13.5 mL of water is added to a graduated cylinder, followed by the pellets. The volume is observed to rise to 15.5 mL. Use Table I below to identify the substance.
15.5 mL - 13.5 mL = 2.0 mL
17.50 g / 2.0 mL = 8.75 g/mL