Match the element names with their symbols
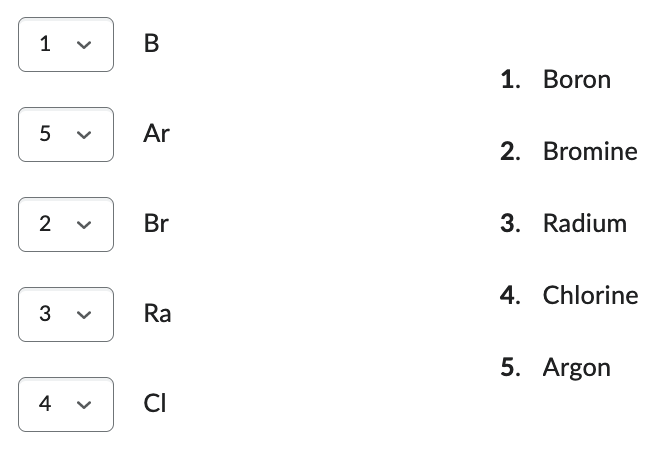
Match the element names with their symbols

What family does calcium belong to on the Periodic Table?
- Halogen
- Nobel Gas
- Alkali Metal
- Transition Metal
- Alkaline Earth Metal
Alkaline Earth Metal
What family does Bromine belong to on the Periodic Table?
- Transition Metal
- Alkali Metal
- Alkaline Earth Metal
- Halogen
- Nobel Gas
Halogen
How many electrons, protons and neutrons are in 1 atom of Tin?
Mass number is 118
1._______ electrons
2._______ protons
3._______ neutrons
1.____50___ electrons
2.____50___ protons
3.____68___ neutrons (118-50= 68)
How many electrons, protons and neutrons are in 1 atom of Uranium?
Mass number is 238
1._______ electrons
2._______ protons
3._______ neutrons
1.____92___ electrons
2.____92___ protons
3.____146___ neutrons (238-92= 146)
When you diagram an atom of silicon you have
______ electrons in the first row,
______ electrons in the second row and
______ electrons in the third row. The answers are all numbers.
___2___ electrons in the first row,
___8___ electrons in the second row and
___4___ electrons in the third row.
Nitrogen has this many valence electrons (answer with a digit). _______
5
Ex. Finding the Number of Valence Electrons for an Element
https://www.youtube.com/watch?v=x1gdfkvkPTk
Fluorine has this many valence electrons (answer with a digit). _______
7
Ex. Finding the Number of Valence Electrons for an Element
https://www.youtube.com/watch?v=x1gdfkvkPTk
Convert 32.00 grams of Ni (OR "N" i) to moles. Do not type units with your answer.
The atomic weight of Ni (OR "N" i) is 58.69 grams/mol
0.5452
Convert 0.532 moles of K to grams of K. Do not type units with your answer.
The atomic weight of K is 39.10 grams/mol
20.8
Convert 393.00 grams of Ba (OR B a) to moles. Do not type units with your answer.
The atomic weight of Ba (OR B a) is 137.33 grams/mol
2.8617
Convert 0.280 moles of Ca (OR C a) to grams. Do not type units with your answer.
The atomic weight of Ca (OR C a) is 40.08 grams/mol
11.2
Convert 98.50 grams of Au (OR A u) to moles. Do not type units with your answer.
The atomic weight of Au (OR A u) is 196.97 grams/mol
0.5001
Assume a hypothetical element X consists of 3 naturally occurring isotopes. The first isotope has a percent abundance of 56.34% and a mass of 45.45 amu. The second isotope has a percent abundance of 30.23% and a mass of 48.37 amu. The third isotope has percent abundance of 13.43% and a mass of 49.81 amu.
What is the average atomic weight of element X? Report your answer to two decimal places and do not include units.
Note: Element X does not exist so you will not find it on the periodic table.
46.92
(45.45)(0.5634)+(48.37)(0.3023)+(49.81)(0.1343)= 46.92
Ex.
Calculate the average atomic weight when given isotopic weights and
abundances
Fifteen Examples
Example #4: How to Calculate an Average Atomic Weight
https://www.chemteam.info/Mole/AverageAtomicWeight.html
Another good one for a similar (NOT THE SAME) problem:
Calculate the isotopic abundances when given the average atomic weight and the isotopic weights
Sixteen Examples
https://www.chemteam.info/Mole/AvgAtomicWt-Reverse.html