When a very electronegative atom strips a valence electron away from its partner, __________ form.
- polar covalent bonds
- covalent bonds
- ions
- hydrogen bonds
- van der Waals interactions
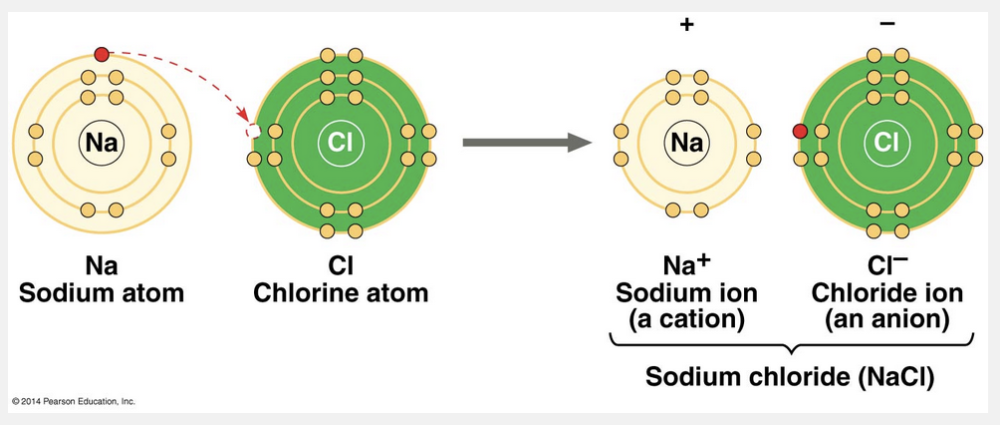
ions
Ex.
When a very electronegative atom strips a valence electron away from its partner, ions form.
In some cases, two atoms are so unequal in their attraction for valence electrons that the more electronegative atom strips an electron completely away from its partner. The two resulting oppositely charged atoms (or molecules) are called ions. A positively charged ion is called a cation, while a negatively charged ion is called an anion. Because of their opposite charges, cations and anions attract each other; this attraction is called an ionic bond. Note that the transfer of an electron is not, by itself, the formation of a bond; rather, it allows a bond to form because it results in two ions of opposite charge. Any two ions of opposite charge can form an ionic bond. The ions do not need to have acquired their charge by an electron transfer with each other.
“Hydrogen bonds” is incorrect because these are weak chemical bonds where there is an attraction between a hydrogen atom and an electronegative atom.
“Van der Waals interactions” is incorrect because this is a weak chemical reaction that occurs only when atoms and molecules are very close together.
“Covalent bonds” is incorrect because this is the sharing of a pair of valence electrons by two atoms.
“Polar covalent bonds” is incorrect because this is the sharing of a pair of valence electrons by two atoms, where one of the atoms is strongly electronegative.
Molecular mimics __________.
- are the receptors for the naturally occurring molecule
- are identical to naturally occurring molecules that are produced by an organism
- destroy the effect of the naturally occurring molecules
- can produce similar effects as the naturally occurring molecules
- None of the listed responses is correct.
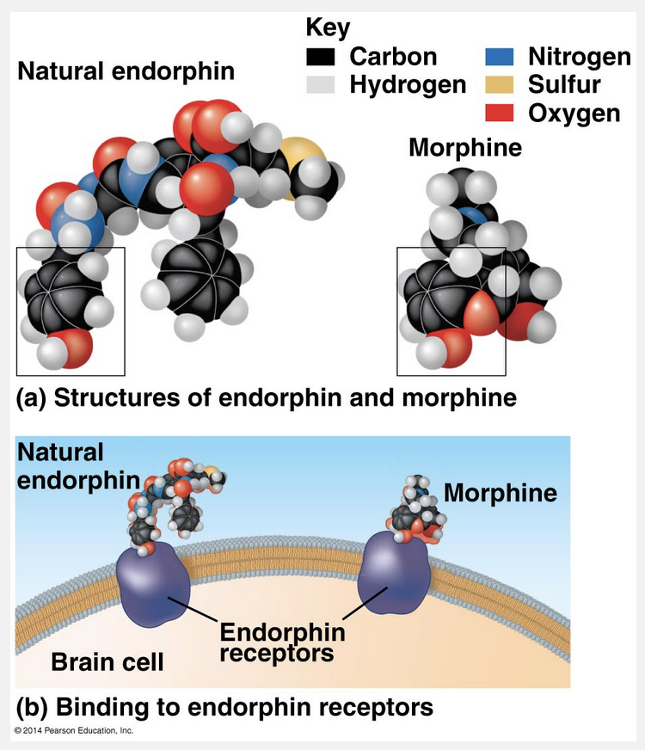
can produce similar effects as the naturally occurring molecules
Ex.
Molecular mimics can produce similar effects as the naturally occurring molecules.
Molecular shape is crucial: It determines how biological molecules recognize and respond to one another with specificity. Biological molecules often bind temporarily to each other by forming weak bonds, but only if their shapes are complementary. Consider the effects of opiates, drugs such as morphine and heroin that are derived from opium. Opiates relieve pain and alter mood by weakly binding to specific receptor molecules on the surfaces of brain cells. Why would brain cells carry receptors for opiates, compounds that are not made by the body? In 1975, the discovery of endorphins answered this question. Endorphins are signaling molecules made by the pituitary gland that bind to the receptors, relieving pain and producing euphoria during times of stress, such as intense exercise. Opiates have shapes similar to that of endorphins and mimic them by binding to endorphin receptors in the brain. That is why opiates and endorphins have similar effects. The role of molecular shape in brain chemistry illustrates how biological organization leads to a match between structure and function, one of biology’s unifying themes.
“Are identical to naturally occurring molecules that are produced by an organism” is incorrect because only a portion of the molecule is identical to the naturally occurring molecule.
“Destroy the effect of the naturally occurring molecules” is incorrect because the mimic mimics the effect of the naturally occurring molecule.
“Are the receptors for the naturally occurring molecules” is incorrect because the mimic binds to the receptor of the naturally occurring molecules and is not the receptor.
In ionic interactions, __________.
- the transfer of valence electrons results in a completed valence shell for each ion
- there is always the equal sharing of electrons
- the cation always gains an electron
- the anion always loses an electron
- there are always weak interactions in a dry salt crystal

the transfer of valence electrons results in a completed valence shell for each ion
Ex.
In ionic interactions, the transfer of valence electrons results in a completed valence shell for each ion.
When two atoms are very unequal in their attraction for valence electrons, the more electronegative atom strips an electron completely away from its partner. The two resulting oppositely charged atoms (or molecules) are called ions. A positively charged ion is called a cation, while a negatively charged ion is called an anion. Because of their opposite charges, cations and anions attract each other; this attraction is called an ionic bond. Note that the transfer of an electron is not, by itself, the formation of a bond; rather, it allows a bond to form because it results in two ions of opposite charge. Any two ions of opposite charge can form an ionic bond. The ions do not need to have acquired their charge by an electron transfer with each other.
“The anion always loses an electron” is incorrect because the anion always gains an electron.
“The cation always gains an electron” is incorrect because the cation always loses an electron.
“There is always the equal sharing of electrons” is incorrect because this is a covalent bond.
“There are always weak interactions in a dry salt crystal” is incorrect because dry salt crystals always have strong interactions.
When one or more pairs of valence electrons are shared by two neutral atoms, what type of bond is formed?
- A hydrogen bond
- An ionic bond
- A covalent bond
- A neutral bond
- An electronegative bond
A covalent bond
Ex.
When one or more pairs of valence electrons are shared by two neutral atoms, a covalent bond is formed. A covalent bond is a chemical bond in which two valence electrons are shared by two neutral atoms, thereby forming a molecule.
An ionic bond, by contrast, is a chemical bond in which an electron is transferred from one atom to another; these bonds occur when one atom is more electronegative than the other and “strips away” an electron from its partner.
A hydrogen bond is the attraction that exists between a hydrogen atom that is already covalently bonded to an electronegative atom, and another electronegative atom.
Neither electronegative nor neutral bonds exist.
The behavior of an atom depends on the __________.
- the atomic mass of the atom
- the electronegativity of the particular atom
- the number of extra neutrons in the nucleus
- valence electrons in the outermost electron shell
- valence electrons in the innermost electron shell
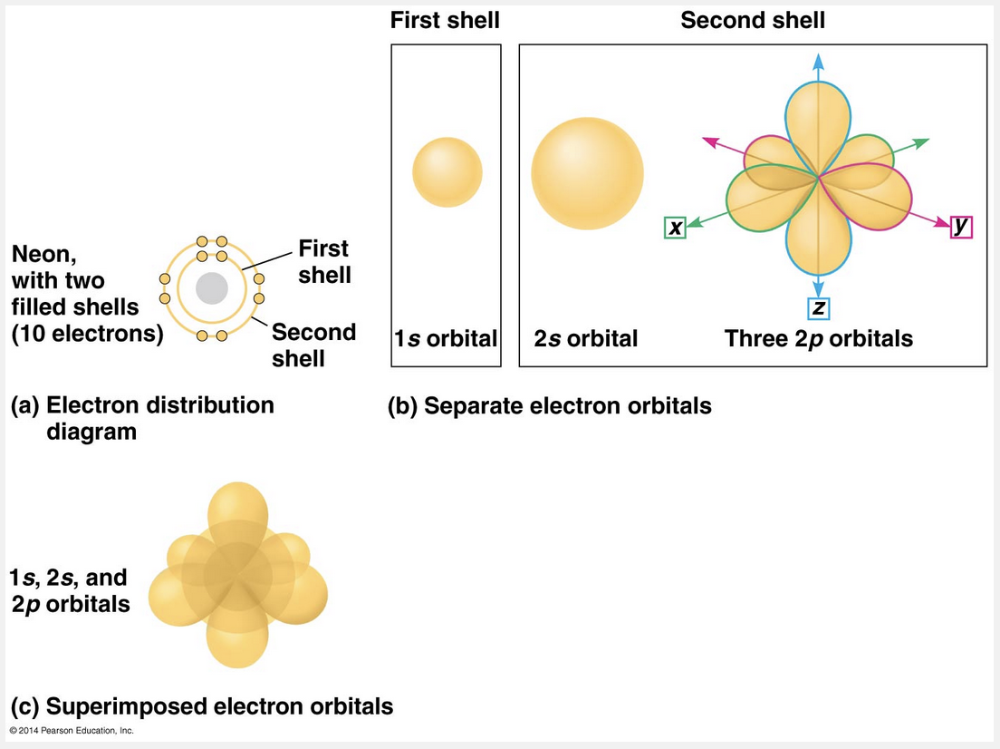
valence electrons in the outermost electron shell
Ex.
The behavior of an atom depends on the valence electrons in the outermost electron shell.
Each electron shell contains electrons at a particular energy level, distributed among a specific number of orbitals of distinctive shapes and orientations. The figure below shows the orbitals of neon as an example, with its electron distribution diagram for reference. You can think of an orbital as a component of an electron shell. The first electron shell has only one spherical s orbital (called 1s), but the second shell has four orbitals: one large spherical s orbital (called 2s) and three dumbbell-shaped p orbitals (called 2p orbitals). No more than 2 electrons can occupy a single orbital. The first electron shell can therefore accommodate up to 2 electrons in its s orbital. The lone electron of a hydrogen atom occupies the 1s orbital, as do the 2 electrons of a helium atom. The four orbitals of the second electron shell can hold up to 8 electrons, 2 in each orbital. Electrons in each of the four orbitals have nearly the same energy, but they move in different volumes of space. The reactivity of an atom arises from the presence of unpaired electrons in one or more orbitals of its valence shell. As you will see, atoms interact in a way that completes their valence shells. When they do so, it is the unpaired electrons that are involved.
“Valence electrons in the innermost electron shell” is incorrect because valence electrons are found in the outermost electron shell.
“The number of extra neutrons in the nucleus” is incorrect because different numbers of neutrons in the nucleus of an atom are isotopes of that atom.
“The electronegativity of the particular atom” is incorrect because valence electrons are found in the outermost electron shell.
“The atomic mass of the atom” is incorrect because of the number of protons and neutrons in the nucleus of an atom.
The reactivity of an atom arises from __________.
- the existence of unpaired electrons in the valence shell
- the potential energy of the valence shell
- the sum of the potential energies of all the electron shells
- the average distance of the outermost electron shell from the nucleus
- the energy difference between the s and p orbitals
the existence of unpaired electrons in the valence shell
Ex.
The reactivity of an atom arises from the existence of unpaired electrons in the valence shell.
It is neither a function of the average distance of the outermost shell from the nucleus, nor of the potential energies of the valence shell or all the electron shells. The atom’s reactivity is not related to the potential energy difference between the s and p orbitals.
Radioactive tracers _________.
- are never hazardous to life because of their usefulness
- are stable isotopes of elements used in medicine
- are important tools in biological research and medicine
- None of the listed responses is correct.
- help locate trace elements on the Earth
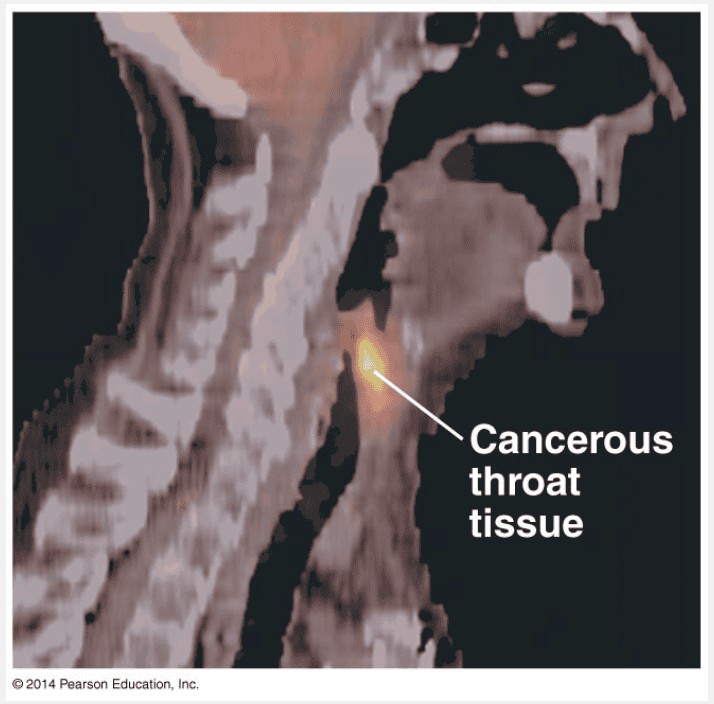
are important tools in biological research and medicine
Ex.
Radioactive tracers are important tools in biological research and medicine.
Radioactive isotopes are often used as diagnostic tools in medicine. Cells can use radioactive atoms just as they would use nonradioactive isotopes of the same element. The radioactive isotopes are incorporated into biologically active molecules, which are then used as tracers to track atoms during metabolism, the chemical processes of an organism. For example, certain kidney disorders are diagnosed by injecting small doses of radioactively labeled substances into the blood and then analyzing the tracer molecules excreted in the urine. Radioactive tracers are also used in combination with sophisticated imaging instruments, such as PET scanners, that can monitor growth and metabolism of cancers in the body. Although radioactive isotopes are very useful in biological research and medicine, radiation from decaying isotopes also poses a hazard to life by damaging cellular molecules. The severity of this damage depends on the type and amount of radiation an organism absorbs. One of the most serious environmental threats is radioactive fallout from nuclear accidents. The doses of most isotopes used in medical diagnosis, however, are relatively safe.
“Help locate trace elements on the Earth” is incorrect because they are used as diagnostic tools in medicine.
“Are never hazardous to life because of their usefulness” is incorrect because radioactive isotopes also pose a hazard to life by damaging cellular molecules.
“Are stable isotopes of elements used in medicine” is incorrect because radioactive tracers are radioactive forms of an atom.
What are the four most abundant elements found in living systems?
- Magnesium, calcium, iron, and zinc
- Hydrogen, oxygen, calcium, and nitrogen
- Nitrogen, carbon, sulfur, and oxygen
- Hydrogen, oxygen, nitrogen, and carbon dioxide
- Hydrogen, oxygen, nitrogen, and carbon
Hydrogen, oxygen, nitrogen, and carbon
Ex.
The four most abundant elements in living systems are hydrogen, oxygen, nitrogen, and carbon, which together make up about 96 percent of the mass of living organisms.
Calcium makes up about 1.5 percent, and sulfur, about 0.3 percent, of living organisms. Magnesium (0.1 percent), iron (<0.01 percent), and zinc (<0.01 percent) are important for life, but make up only a small percentage of body mass.
Carbon dioxide is a compound, not an element.
In chemical reactions, the __________ are the starting materials that are converted into the __________.
- reactants; products
- products; reactants
- None of the listed responses is correct.
- reactants; un-rearranged matter
- reactants; destroyed atoms
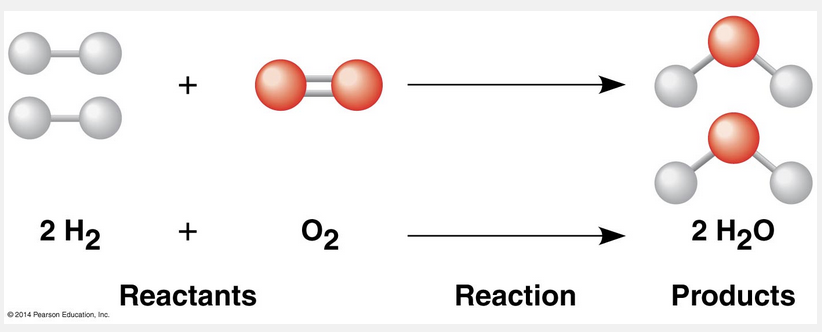
reactants; products
Ex.
In chemical reactions, the reactants are the starting materials that are converted into the products.
The making and breaking of chemical bonds, leading to changes in the composition of matter, are called chemical reactions. An example is the reaction between hydrogen and oxygen molecules that forms water: This reaction breaks the covalent bonds of H2 and O2 and forms the new bonds of H2O. When we write a chemical reaction, we use an arrow to indicate the conversion of the starting materials, called the reactants, to the products. The coefficients indicate the number of molecules involved; for example, the coefficient 2 in front of the H2 means that the reaction starts with two molecules of hydrogen. Notice that all atoms of the reactants must be accounted for in the products. Matter is conserved in a chemical reaction: Reactions cannot create or destroy atoms but can only rearrange (redistribute) the electrons among them.
“Products; reactants” is incorrect because the reactants are always the starting materials.
“Reactants; un-rearranged matter” is incorrect because chemical reactions lead to changes in the composition of matter.
“Reactants; destroyed atoms” is incorrect because reactions cannot create or destroy atoms but can only rearrange (redistribute) the electrons among them.
Nonpolar covalent bonds are always formed from atoms that are __________.
- equal in their electronegativity
- unequal in their electronegativity, with one atom being very strongly electronegative
- both strongly and weakly electronegative
- both strongly and somewhat strongly electronegative
- None of the listed responses is correct.
equal in their electronegativity
Ex.
Nonpolar covalent bonds are always formed from atoms that are equal in their electronegativity.
Atoms in a molecule attract shared bonding electrons to varying degrees, depending on the element. The attraction of a particular atom for the electrons of a covalent bond is called its electronegativity. The more electronegative an atom is, the more strongly it pulls shared electrons toward itself. In a covalent bond between two atoms of the same element, the electrons are shared equally because the two atoms have the same electronegativity—the tug-of-war is at a standoff. Such a bond is called a nonpolar covalent bond.
“Both strongly and somewhat strongly electronegative” and “both strongly and weakly electronegative” are both incorrect because nonpolar covalent bonds are formed from atoms that are equal in their electronegativity.
“Unequal in their electronegativity, with one atom being very strongly electronegative,” is incorrect because this would result in a polar covalent bond.
The chemical characteristics or reactivity of an element depend mostly on the __________.
- degree to which it has more or fewer electrons than protons
- mean energy level of its electrons
- number of protons plus the number of neutrons
- number of electron shells present in the atoms
- number of electrons in its outermost shell
number of electrons in its outermost shell
Ex.
The chemical characteristics or reactivity of an element depend mostly on the number of electrons in its outermost shell.
The chemical characteristics and reactivity of an element depend mainly on the number of electrons in its atoms’ outermost valence shell. These are the electrons that are most free to interact chemically with the electrons of other atoms.
The number of electron shells is not an important determinant of an atom’s reactivity.
The number of protons plus the number of neutrons is the element’s mass number; it does not determine the element’s reactivity.
The mean energy level of the electrons is not important in determining the atom’s reactivity.
The difference in the number of protons versus electrons affects the atom’s charge, but not its reactivity.
Trace elements __________.
- are generally found in small quantities in the earth and are also called rare elements
- are needed for detecting organisms in the environment
- are required by an organism in minute quantities
- None of the listed responses is correct.
- are primarily used for making silicon chips for computers
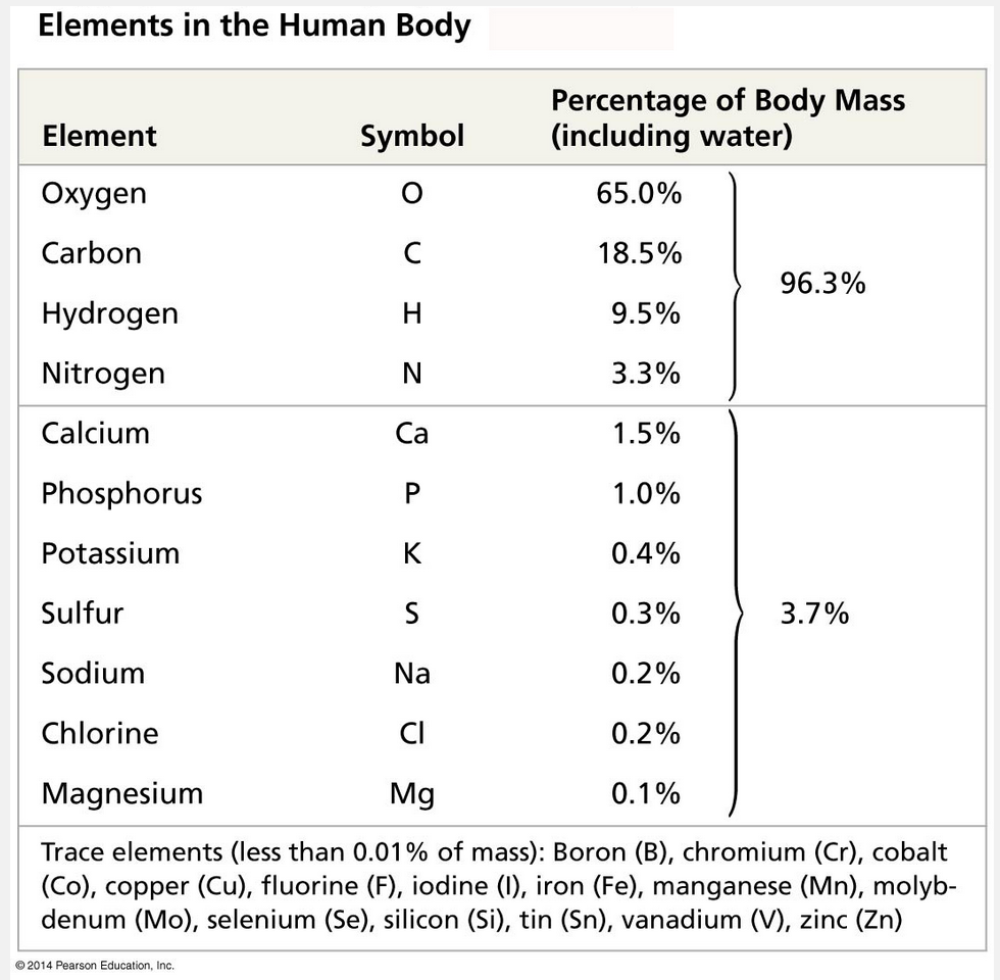
are required by an organism in minute quantities
Ex.
Trace elements are required by an organism in minute quantities.
Some trace elements, such as iron (Fe), are needed by all forms of life; others are required only by certain species. For example, in vertebrates (animals with backbones), the element iodine (I) is an essential ingredient of a hormone produced by the thyroid gland. A daily intake of only 0.15 milligram (mg) of iodine is adequate for normal activity of the human thyroid. An iodine deficiency in the diet causes the thyroid gland to grow to abnormal size, a condition called goiter. Where it is available, eating seafood or iodized salt reduces the incidence of goiter. All the elements needed by the human body are listed in the table below.
“Are needed for detecting organisms in the environment,” “are generally found in small quantities in the earth and are also called rare elements,” and “are primarily used for making silicon chips for computers” are incorrect because trace elements are needed by all forms of life.
Isotopes of an element will always differ in __________.
- atomic number
- number of protons
- number of electrons
- atomic mass
atomic mass
Ex.
Isotopes of an element will always differ in atomic mass.
An element’s atomic number is the number of protons, whereas its atomic mass is the sum of the number of protons and neutrons, in the nucleus.
The isotopes of an element have the same number of protons and therefore the same atomic number; however, they have different numbers of neutrons and therefore differ in atomic mass.
Elements with the same number of protons but different numbers of electrons carry a charge; they are called ions of the element.
The number of protons in an uncharged atom __________.
- varies with the different isotopes
- equals the number of electrons
- equals the number of neutrons
- determines its mass number
- equals the number of electrons in the outer orbital of the atom
equals the number of electrons
Ex.
The number of protons in an uncharged atom equals the number of electrons.
An uncharged atom is an atom that lacks an electrical charge: It is electrically neutral. Neutrons are electrically neutral, and therefore the number of neutrons do not contribute to the atom’s charge. An uncharged atom must have an equal number of protons, which carry a positive charge, and electrons, which carry a negative charge.
Isotopes of an element have the same number of protons and electrons, but different numbers of neutrons; all isotopes therefore carry the same charge. The number of protons and the total number of electrons, not just the number in the outer orbital, are equal in an uncharged atom.
The number of protons determines an element’s atomic number, not the mass number, which equals the sum of the number of protons and the number of neutrons.
The difference between a stable isotope and an unstable isotope of an element is that __________.
- the stable isotope has a nucleus that decays spontaneously but is not radioactive
- the unstable isotope decays and is always transformed into a stable form of the same element
- the unstable isotope has a nucleus that decays spontaneously, is radioactive, and can be transformed into an atom of another element
- the stable isotope has a nucleus that decays spontaneously and is radioactive
- the unstable isotope has a nucleus that does not decay spontaneously and is never radioactive
the unstable isotope has a nucleus that decays spontaneously, is radioactive, and can be transformed into an atom of another element
Ex.
The difference between a stable isotope and an unstable isotope of an element is that the unstable isotope has a nucleus that decays spontaneously, is radioactive, and can be transformed into an atom of another element.
All atoms of a given element have the same number of protons, but some atoms have more neutrons than other atoms of the same element and therefore have greater mass. These different atomic forms of the same element are called isotopes of the element. In nature, an element occurs as a mixture of its isotopes. As an explanatory example, let’s consider the three naturally occurring isotopes of the element carbon, which has the atomic number 6. The most common isotope is carbon-12, 12 6C, which accounts for about 99% of the carbon in nature. The isotope 12 6C has 6 neutrons. Most of the remaining 1% of carbon consists of atoms of the isotope 13 6C, with 7 neutrons. A third, even rarer isotope, 14 6C, has 8 neutrons. Both 12C and 13C are stable isotopes, meaning that their nuclei do not have a tendency to lose subatomic particles, a process called decay. The isotope 14C, however, is unstable, or radioactive. A radioactive isotope is one in which the nucleus decays spontaneously, giving off particles and energy. When the radioactive decay leads to a change in the number of protons, it transforms the atom to an atom of a different element. For example, when an atom of carbon-14 (14C) decays, it becomes an atom of nitrogen (14N). Radioactive isotopes have many useful applications in biology.
“The unstable isotope has a nucleus that does not decay spontaneously and is never radioactive” and “the unstable isotope decays and is always transformed into a stable form of the same element” are both incorrect because the unstable isotope always decays spontaneously and is always transformed into another element.
“The stable isotope has a nucleus that decays spontaneously and is radioactive” is incorrect because the stable isotope does not decay spontaneously and is not radioactive.
“The stable isotope has a nucleus that decays spontaneously but is not radioactive” is incorrect because the stable isotope does not decay spontaneously.
Orbitals are best described as __________.
- a three-dimensional space where electrons and neutrons interact with each other
- a three-dimensional space where an electron spends most of its time with at least 3 other electrons
- a three-dimensional space where electrons and protons interact with each other
- a three-dimensional space where an electron spends most of its time with no more than 1 other electron, forming a pair
- a shell that can hold any number of electrons

a three-dimensional space where an electron spends most of its time with no more than 1 other electron, forming a pair
Ex.
Orbitals are best described as a three-dimensional space where an electron spends most of its time with no more than 1 other electron, forming a pair.
The three-dimensional space where an electron is found 90% of the time is called an orbital. Each electron shell contains electrons at a particular energy level, distributed among a specific number of orbitals of distinctive shapes and orientations. The figure below shows the orbitals of neon as an example, with its electron distribution diagram for reference. You can think of an orbital as a component of an electron shell. The first electron shell has only one spherical s orbital (called 1s), but the second shell has four orbitals: one large spherical s orbital (called 2s) and three dumbbell-shaped p orbitals (called 2p orbitals). No more than 2 electrons can occupy a single orbital. The first electron shell can therefore accommodate up to 2 electrons in its s orbital. The lone electron of a hydrogen atom occupies the 1s orbital, as do the 2 electrons of a helium atom. The four orbitals of the second electron shell can hold up to 8 electrons, 2 in each orbital. Electrons in each of the four orbitals have nearly the same energy, but they move in different volumes of space. The reactivity of an atom arises from the presence of unpaired electrons in one or more orbitals of its valence shell. As you will see, atoms interact in a way that completes their valence shells. When they do so, it is the unpaired electrons that are involved.
“A three-dimensional space where an electron spends most of its time with at least 3 other electrons” is incorrect because no more than 2 electrons can occupy a single orbital. “A three-dimensional space where electrons and protons interact with each other” is incorrect because protons are found in the nucleus of the atom and not in the orbitals. “As a shell that can hold any number of electrons” is incorrect because no more than 2 electrons can occupy a single orbital regardless of how many orbitals a shell has. “A three-dimensional space where electrons and neutrons interact with each other” is incorrect because neutrons are found in the nucleus of the atom and not in the orbitals.
Ionic bonds form as a result of __________.
- attraction between ions that have opposite charges
- unequal sharing of electrons between atoms
- the asymmetric distribution of electrons in constant motion
- sharing of electron pairs between atoms
- attraction between hydrogen and other atoms that share electrons unequally
attraction between ions that have opposite charges
Ex.
Ionic bonds form as a result of attraction between ions that have opposite charges.
Ionic bonds are attractions that form between ions of opposite charges. In an ionic bond, electrons are transferred from one ion (the cation) to the other (the anion), not shared, as is the case for covalent bonds.
A hydrogen bond is formed as a result of noncovalent attraction between a hydrogen atom and an electronegative atom.
Unequal sharing of electrons occurs in a polar covalent bond.
Van der Waals bonds involve ever-changing regions of positive and negative charge that allow atoms and molecules to stick to one another.
Which of the following four statements, if any, is true regarding essential elements and living organisms?
- All of the listed responses are true.
- The elements carbon, hydrogen, nitrogen, and oxygen make up 20–25 percent of living mass.
- All organisms require 25 of the 92 naturally occurring elements to survive.
- Although all forms of life require iron, other elements are required only by certain species.
- Given their low concentrations in nature, the toxicity of some elements is generally not a factor in the evolution of biological communities.
Although all forms of life require iron, other elements are required only by certain species.
Ex.
The following statement is true: Although all forms of life require iron, other elements are required only by certain species.
Species differ in the number of essential elements they require: Humans need 25, but plants require only 17. Carbon, hydrogen, nitrogen, and oxygen make up a large proportion—about 96 percent—of living matter. All forms of life require iron, whereas other elements are needed by certain species only; for example, humans and vertebrates need iodine, but invertebrates do not. Toxic elements can play an important role in evolution, as in the case of the plants that have adapted to survive in serpentine soil, which has high levels of toxic elements like chromium, nickel, and cobalt.
The molecular shape of a biological molecule is important because __________.
- it is never important since cells react equally to the different molecular shapes of the biological molecule
- None of the listed responses is correct.
- it determines the one-dimensional shape of the molecule
- it determines how biological molecules recognize and respond to one another
- it helps determine if the molecule is ionic or covalent

it determines how biological molecules recognize and respond to one another
Ex.
The molecular shape of a biological molecule is important because it determines how biological molecules recognize and respond to one another.
Molecular shape is crucial and determines how biological molecules recognize and respond to one another with specificity. Biological molecules often bind temporarily to each other by forming weak bonds, but only if their shapes are complementary. Consider the effects of opiates, drugs such as morphine and heroin that are derived from opium. Opiates relieve pain and alter mood by weakly binding to specific receptor molecules on the surfaces of brain cells. Why would brain cells carry receptors for opiates, compounds that are not made by the body? In 1975, the discovery of endorphins answered this question. Endorphins are signaling molecules made by the pituitary gland that bind to the receptors, relieving pain and producing euphoria during times of stress, such as intense exercise. Opiates have shapes similar to those of endorphins and mimic them by binding to endorphin receptors in the brain. That is why opiates and endorphins have similar effects. The role of molecular shape in brain chemistry illustrates how biological organization leads to a match between structure and function, one of biology’s unifying themes.
“It determines the one-dimensional shape of the molecule” is incorrect because the molecular shape involves the three-dimensional shape of a molecule.
“It helps determine if the molecule is ionic or covalent” is incorrect because molecular shape is crucial in determining how biological molecules recognize and respond to one another with specificity.
“It is never important since cells react equally to the different molecular shapes of the biological molecule” is incorrect because molecular shape is crucial in determining how biological molecules recognize and respond to one another with specificity.
Hydrogen bonds occur when __________.
- two atoms achieve stable electron configurations by sharing electrons with each other
- a molecule with a low molecular weight is bonded to a molecule with a high molecular weight
- a molecule with partial charges contacts a molecule without partial charges
- None of the listed responses is correct.
- partial opposite charges on molecules come close enough to attract each other
partial opposite charges on molecules come close enough to attract each other
Ex.
Hydrogen bonds occur when partial opposite charges on molecules come close enough to attract each other.
Hydrogen bonds are noncovalent bonds that result from an attraction between a hydrogen molecule that is already covalently bonded to an electronegative atom, and another electronegative atom. Hydrogen bonds thus form between molecules with partial opposite charges.
The molecular weight of the atoms involved is not relevant for hydrogen bonding. Electrons are shared in covalent bonds, but not in hydrogen bonds.
Which of the following subatomic particles has appreciable mass and lacks a charge?
- Proton
- Element
- Neutron
- Electron
- Molecule
Neutron
Ex.
A neutron has appreciable mass and lacks a charge.
Only two subatomic particles have appreciable (“large”, opposite of “negligible” in previous question) mass, the proton and the neutron. You can get a hint about charge from the name: a “neutron” is “neutral”, meaning that it lacks a charge and is the correct answer.
Although protons have a substantial mass (about 1 dalton, similar to neutrons), they carry a positive charge.
Electrons have a negative charge and a negligible mass (about 1/2,000 the mass of a proton).
An element is not a subatomic particle: It is made of atoms, which in turn are made of subatomic particles.
A molecule is not a subatomic particle: It consists of two or more elements joined by covalent bonds.
Which statement is true of all atoms that are anions?
- The atom has more neutrons than protons.
- The net charge is -1.
- The atom has more electrons than protons.
- The atom has more protons than electrons.
- The atom has fewer protons than does a neutral atom of the same element.
The atom has more electrons than protons.
Ex.
The atom has more electrons than protons is true of all atoms that are anions.
This is a definition question. Remember that protons carry a +1 charge, electrons carry a -1 charge, and neutrons carry no charge. A cation is an atom that has more protons than electrons and carries a positive charge (think of the “t” in “cation”: it looks like a “+” sign). An anion is an atom that has more electrons than protons and carries a negative charge. Anions do not always have negative charges of -1: the number of electrons can exceed the number of protons by more than 1. An atom with fewer protons than the neutral atom of the same element would be a different element. Having more neutrons than protons would not affect the charge on the atom.
When the proton number and electron number are unequal, the atom or molecule __________.
- gains or loses a neutron
- is an ion
- forms a covalent bond with another atom
- becomes part of a molecule
- gains or loses a proton
is an ion
Ex.
When the proton number and electron number are unequal, the atom or molecule is an ion.
Different numbers of protons and electrons in an atom result in the atom having an electrical charge. The charge is either positive or negative, depending on whether there are more protons or more electrons, and such an atom is called an ion.
Covalent bonds form when electrons are shared, not when there are different numbers of protons and electrons.
An atom becomes part of a molecule when it covalently bonds to another atom by sharing electrons.
The gain or loss of a proton would cause the atom to become a different element.
Which of the following has negligible mass?
- Atom
- Neutron
- Proton
- Element
- Electron
Electron
Ex.
An electron has negligible mass. “Negligible” mass means a mass so low that it is not taken into account in ordinary calculations of atomic mass. The subatomic particle that fits this description is the electron. Its mass is only about 1/1,200 the mass of a proton or neutron.
Both atoms and elements are important components of atomic mass and weigh about 1.7 x 1024 grams, equal to about 1 dalton, the standard unit of measurement of atomic mass. The atomic mass of an element is equal to the sum of the masses of its protons and its neutrons. Atoms are made of subatomic particles (protons, neutrons, and electrons).
The fact that oxygen is an atom that is strongly electronegative means that __________.
- it equally pulls shared electrons toward itself when forming a covalent bond with a less electronegative atom
- the bond formed will always be ionic since there is unequal sharing
- the bond formed will be a nonpolar covalent bond
- it more strongly pulls shared electrons toward itself when forming a covalent bond with a less electronegative atom
- the less electronegative atom will pull the electrons more strongly than oxygen will
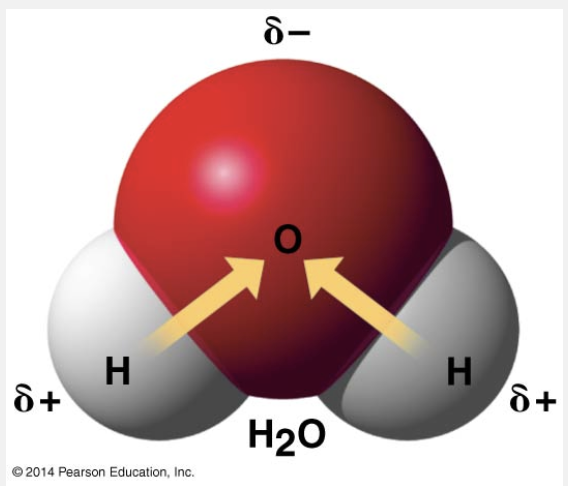
it more strongly pulls shared electrons toward itself when forming a covalent bond with a less electronegative atom
Ex.
The fact that oxygen is an atom that is strongly electronegative means that it more strongly pulls shared electrons toward itself when forming a covalent bond with a less electronegative atom.
Atoms in a molecule attract shared bonding electrons to varying degrees, depending on the element. The attraction of a particular atom for the electrons of a covalent bond is called its electronegativity. The more electronegative an atom is, the more strongly it pulls shared electrons toward itself. Oxygen is one of the most electronegative elements, attracting shared electrons much more strongly than hydrogen does. In a covalent bond between oxygen and hydrogen, the electrons spend more time near the oxygen nucleus than they do near the hydrogen nucleus.
“It equally pulls shared electrons toward itself when forming a covalent bond with a less electronegative atom” is incorrect because when oxygen forms a bond with a less electronegative atom, the electrons spend more time near the oxygen nucleus.
“The less electronegative atom will pull the electrons more strongly than oxygen will” is incorrect because the more electronegative atom, in this case oxygen, will pull the electrons more strongly.
“The bond formed will always be ionic since there is unequal sharing” is incorrect because ionic bonds are charge interactions and not shared interactions.
“The bond formed will be a nonpolar covalent bond” is incorrect because when a strongly electronegative atom forms a covalent bond with a weakly electronegative atom, a polar covalent bond forms.
A covalent bond __________.
- always forms a crystal salt
- is the sharing of a pair of valence electrons by two atoms
- is always one more bond than the valence number for the pair
- is a weak chemical bond
- is the transfer of a valence electron to an atom that has an unfilled valence shell
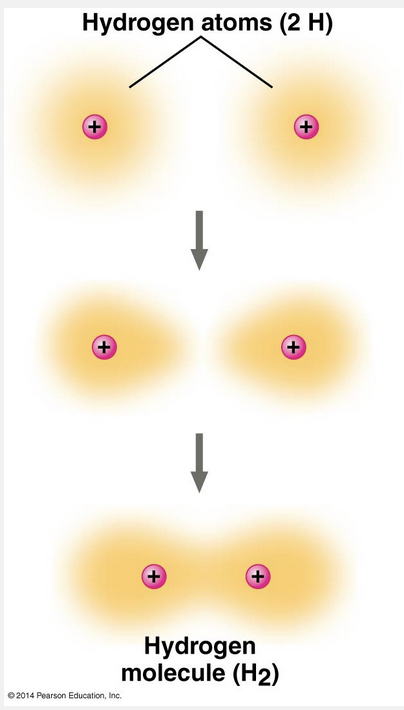
is the sharing of a pair of valence electrons by two atoms
Ex.
A covalent bond is the sharing of a pair of valence electrons by two atoms.
For example, let’s consider what happens when two hydrogen atoms approach each other. Recall that hydrogen has 1 valence electron in the first shell, but the shell’s capacity is 2 electrons. When the two hydrogen atoms come close enough for their 1s orbitals to overlap, they can share their electrons. Each hydrogen atom is now associated with 2 electrons in what amounts to a completed valence shell. Two or more atoms held together by covalent bonds constitute a molecule, in this case a hydrogen molecule. The strongest kinds of chemical bonds are covalent bonds and ionic bonds (when in dry ionic compounds).
“is the transfer of a valence electron to an atom that has an unfilled valence shell” is incorrect because this describes an ionic bond.
“Is always one more bond than the valence number for the pair” is incorrect because each bond is formed by the sharing of a pair of valence electrons by two atoms.
“Is a weak chemical bond” is incorrect because covalent bonds are strong bonds.
“Always forms a crystal salt” is incorrect because salts are formed from ionic interactions.
In a process called radiometric dating, scientists measure the __________ of an isotope to determine the age of the Earth.
- potential energy
- mass number
- daltons
- half-life
- number of valence electrons
half-life
Ex.
In a process called radiometric dating, scientists measure the half-life of an isotope to determine the age of the Earth.
Researchers use radioactive isotopes to date relics of past life. A radioactive isotope is one in which the nucleus decays spontaneously, giving off particles and energy. When the radioactive decay leads to a change in the number of protons, it transforms the atom to an atom of a different element. A “parent” isotope decays into its “daughter” isotope at a fixed rate, expressed as the half-life of the isotope—the time it takes for 50% of the parent isotope to decay. Each radioactive isotope has a characteristic half-life that is not affected by temperature, pressure, or any other environmental variable. Using a process called radiometric dating, scientists measure the ratio of different isotopes and calculate how many half-lives (in years) have passed since an organism was fossilized or a rock was formed. Half-life values range from very short for some isotopes, measured in seconds or days, to extremely long for other isotopes—for example, uranium-238 has a half-life of 4.5 billion years! Each isotope can best “measure” a particular range of years: Uranium-238 was used to determine that moon rocks are approximately 4.5 billion years old, similar to the estimated age of Earth.
“Number of valence electrons” is incorrect because these are unpaired electrons in the outermost shell of an atom.
“Mass number” is incorrect because this is the number of protons and neutrons in an atom’s nucleus.
“Potential energy” is incorrect because this is the energy that matter possesses as a result of its location or spatial arrangement or structure.
“Daltons” is incorrect because this is a measure of mass for atoms and subatomic particles.
Which of the following statements is true about chemical reactions?
- They may have different numbers of a given atom on each side of the equation arrow.
- Only inorganic molecules can participate in chemical reactions.
- They involve the making and breaking of chemical bonds.
- They reach chemical equilibrium when the amounts of products and reactants are equal.
- They represent the way matter is created and destroyed.
They involve the making and breaking of chemical bonds.
Ex.
The following statement is true about chemical reactions: They involve the making and breaking of chemical bonds.
Chemical reactions lead to changes in the composition of matter by making and breaking chemical bonds.
Matter is rearranged, but is neither created nor destroyed, in ordinary chemical reactions.
Chemical equilibrium exists when the forward and reverse rates of the reaction are equal; it does not depend on the amounts or concentrations of reactants and products.
Because matter is being neither created nor destroyed, the number of particular atoms on either side of the reaction equation is always the same.
Any reactive atom or molecule that can react, not just organic molecules, can participate in a chemical reaction.
A covalent bond is likely to be polar if __________.
- the two atoms sharing electrons are equally electronegative
- it is between two atoms that are both very strong electron donors
- the two atoms sharing electrons are of the same element
- one of the atoms sharing electrons is much more electronegative
- it is between two atoms that are both very strong electron acceptors
one of the atoms sharing electrons is much more electronegative
Ex.
A covalent bond is likely to be polar if one of the atoms sharing electrons is much more electronegative.
In a polar covalent bond, one of the atoms in the bond is much more electronegative than the other and therefore “pulls” the shared electrons to it. This is “polarity.”
If the atoms sharing electrons are equally electronegative, the electrons are shared equally and the bond is nonpolar. This would be the case, for example, for two atoms of the same element.
Two strong electron acceptors or electron donors would not necessarily form a polar covalent bond; it is the difference in electronegativity between two atoms that determines polarity.
Weak chemical bonds such as hydrogen bonds __________.
- form when a hydrogen atom that is covalently bonded to an electronegative atom like oxygen or nitrogen is attracted to another electronegative atom
- form when an oxygen atom that is covalently bonded to an electronegative atom like hydrogen is attracted to another electronegative atom
- are not important in biological systems
- form when a hydrogen atom that is covalently bonded to a weakly electronegative atom like oxygen or nitrogen is attracted to another weakly electronegative atom
- None of the listed responses is correct.
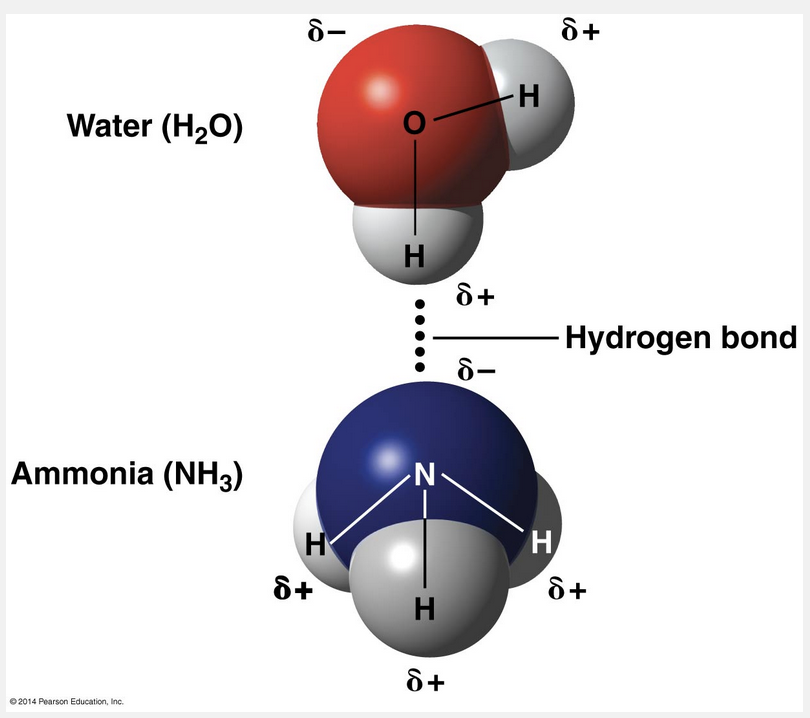
form when a hydrogen atom that is covalently bonded to an electronegative atom like oxygen or nitrogen is attracted to another electronegative atom
Ex.
Weak chemical bonds such as hydrogen bonds form when a hydrogen atom that is covalently bonded to an electronegative atom like oxygen or nitrogen is attracted to another electronegative atom.
Among weak chemical bonds, hydrogen bonds are so central to the chemistry of life that they deserve special attention. When a hydrogen atom is covalently bonded to an electronegative atom, the hydrogen atom has a partial positive charge that allows it to be attracted to a different electronegative atom nearby. This attraction between a hydrogen and an electronegative atom is called a hydrogen bond. In living cells, the electronegative partners are usually oxygen or nitrogen atoms.
“Are not important in biological systems” is incorrect because hydrogen bonds are important in biological systems.
“Form when an oxygen atom that is covalently bonded to an electronegative atom like hydrogen is attracted to another electronegative atom” is incorrect because hydrogen is not an electronegative atom.
“Form when a hydrogen atom that is covalently bonded to a weakly electronegative atom like oxygen or nitrogen is attracted to another weakly electronegative atom” is incorrect because both oxygen and nitrogen are strongly electronegative atoms.