Which substance is a stronger acid; H2SO4(pKa-4) or HCl (pKa-3)?
: H2SO4 because the pKa is lower, it will more readily donate a hydrogen...even though pure versions of both substances will readily burn your skin off
What are the way CO2 can travel around the body?
1. Dissolved in the plasma
2. Carried in the RBC
a. Bound
to hemoglobin (carboxyhemoglobin)
b. Dissolved inside
erythrocytes
3. Dissolved bicarbonate (HCO3) in the plasma
transforms into carbonic acid (H2CO3)
A 15-year-old emotional female comes into the ER after ingesting a bottle full of Aspirin (Acetylsalicylic Acid). She presents with hyperventilation and tinnitus (ringing in the ears). How will she rid her body of the excess hydrogens supplied by the Aspirin?
She will breathe it out because her carbonic anhydrase will combine the excess hydrogens with bicarbonate to create carbon dioxide to be breathed out. Unfortunately, Aspirin overdose will also have other undesirable side effects.
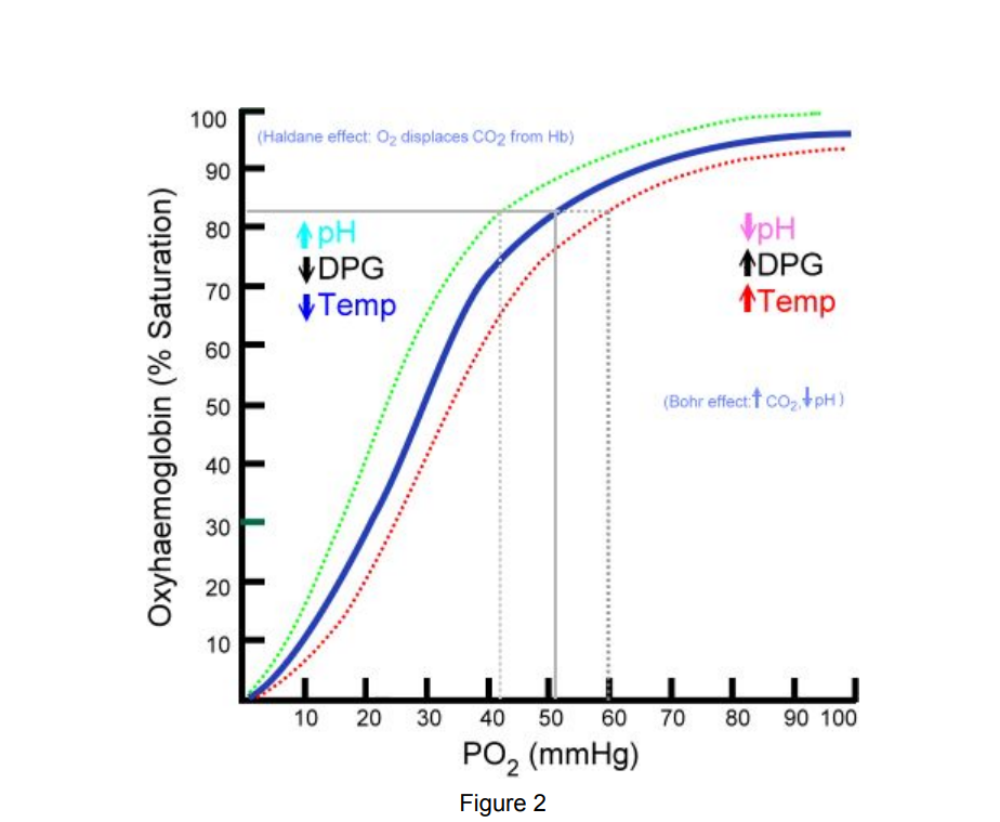
Which variable cause a right shift that will favor oxygen?
● Increased CO2
●
Acidosis
● Increased DPG
●
Exercise
● Increased Temperature
note: “CADET, face Right!”
An athletic 19-year-old private first class in the US Army is running his 1.5 mile PT test. He is trying to get under 9 minutes. What factors will facilitate the unloading of oxygen to his heart and legs during this test?
Answer: CO2, Acidosis, Exercise, and Increased temperature
An 8-year-old boy is noticed by his mother to be heading to the
bathroom every 30 minutes. He also has tried to make toast for
breakfast using the microwave. He said, “that was dumb” when he
realized his mistake. What would you expect this boy’s
serum
potassium level to be less than?
Answer: 3.5 mg/dL - the reference range for serum potassium is 3.5-5.0 mg/dL
note: This means that much of the intracellular potassium will be excreted in the urine
While assessing the ions in a solution, the laboratory technician
notices that the HCO3 concentration is 20 times higher than the
concentration of H2CO3 The pKa of H2CO3 is 6.35.
What is the pH
of the solution?
A. 7.25
B. 7.45
C. 7.65
D. 7.85
The correct answer is C. The Henderson-Hasselbalch equation is used
to figure this out.
pH = pKa + log (Base/Acid)
pH = 6.35 +
log (20/1)
pH = 6.35 + 1.30
pH = 7.65
This pH is close to the homeostatic physiologic state. While the carbonic acid buffer is one of the most prevalent in our bodies, it is by no means the only buffer system in our body. The rest of the acidity comes from the proteins and other factors to bring the pH closer to 7.4.
. A 37-year-old male smoker is having difficulty breathing. It is
known that 2,3-DPG is significantly decreased in smokers versus
nonsmokers. What effect does this low concentration have on the
patient’s hemoglobin to carry oxygen?
A. Favors oxygen unloading
into the tissues
B. Favors oxygen retention in the
tissues
C. Favors the oxidation of hemoglobin
D. Favors the
reduction of hemoglobin
The correct answer is B. Increased 2,3-DPG concentration leads to a
“right-shift”, therefore if it’s lacking, then we have a “left shift”
of the curve. This leads to oxygen retention on hemoglobin and allows
for more hemoglobin to be saturated at a specific partial pressure of
Oxygen.
Remember the mnemonic “CADET face right!
A 67-year-old male was arrested in Georgia for bootlegging liquor.
Upon questioning, he said that, “he never tried the stuff anymore,
because it made him feel too loopy and lightheaded.” It was later
found that his liquor contained enough methanol to cause substantial
harm to anyone who drank a substantial amount of it. Many of those who
imbibed on his moonshine, ended up in metabolic acidosis. In what form
would the excess H+ be removed from the body?
A. CO2
B.
H+
C. HCO3-
D. H2CO3
The correct answer is A. Remember the enzyme carbonic anhydrase and how it can interconvert CO2 , H2O, H2CO3, H+, and HCO3. Each one of those molecules can transform into another quite rapidly. Metabolic acidosis is an excess of H+ in the body. The HCO3- will bind to the H+ to form H2CO3 , which will then be turned into carbon dioxide and water to be excreted.
. A 16-year-old girl is having an anxiety attack. She is tachycardic
(fast heart rate), diaphoretic (sweating), and hyperventilating
(breathing quickly). Because the hyperventilation gets rid of a lot of
CO2
, what would you expect her Cl- level to be?
A.
High
B. Low
C. Normal
D. There is no relationship
between acid-base balance and chloride
The correct answer is A. We would expect her chloride level to be
elevated. This young girl is breathing rapidly which efficiently
removes CO2 from the lungs. With such rapid breathing, you
can
blow off so much CO2 that you become alkalemic. This is called
respiratory alkalosis and occurs with hyperventilation. The treatment
for hyperventilation is to breathe into a bag. You breathe back in the
same CO2 that you breathed out, so it helps you not become so
alkalotic. Because this girl is already alkalotic, the bicarbonate
(HCO3-) in her blood will be exchanged for Cl -in the RBCs. This will
cause an increase in the extracellular Chloride concentration
(hyperchloremia)
A 37-year-old professional football player overdosed on opiates prescribed by his physician for a broken femur. He presents to the emergency room in an ambulance and he is found to be apneic (not breathing). He has a tachycardia with a faint pulse and the patient is on a bag-valve mask with 100% O2. His physician wants to check blood gases to ensure adequate oxygenation. What electrode will the laboratory technicians use to assess pO2?
Answer:
Clarke Electrode, which is amperometric and measures the
current produced after being exposed to a standardized potential
between two electrodes.
What are the blood gas ranges you need to know?
pH: 7.35-7.45
pCO2: 34-45 mm/Hg
pO2: 90-100 mm/Hg
HCO3: 22-28 mEq/L
What is the only value on the ABG that is calculated and NOT directly
measured?
HCO3 - is calculated from the Henderson-Hasselbalch equation using pCO2 as the acid species, a pKa of 6.4 for H2CO3, and the pH. I don’t think that you’ll be required to calculate it, but you will NEED to know that HCO3 - is CALCULATED and NOT directly measured.
What is the variable which is altered in respiratory acid-base disorders?
pCO2 is decreased in hyperventilation. With more breaths per minute,
more CO2 is removed from the blood. pCO2 is increased in many other
pulmonary disorders which impair gas exchange in the pulmonary
capillaries (e.g. emphysema, atelectasis [collapsed alveoli],
pneumonia, Acute Respiratory Distress Syndrome
[ARDS], pulmonary
edema, etc.).
How is the primary cause of alkalosis determined?
With an alkalotic pH, either pCO2 is decreased or HCO3 is increased. Usually, whichever one deviates in the direction that would cause the pH change is the primary cause
What is hyperventilation cause?
.
respiratory alkalosis because the person is breathing so quickly and has adequate gas exchange, so they are breathing off all of their CO2
What are examples of Respiratory Alkalosis?
1. High pH
2. Low pCO2
3. Variable HCO3- (due to
compensation and chronicity)
4. Is the pH in the 7.35-7.45 window?
What do the following diseases have in common: Emphysema, Pneumonia, Bronchiectasis, Atelectasis, ARDS, Pulmonary Edema, etc
respiratory acidosis because of the inefficient gas exchange inside the lungs. These patients may be short of breath and breathing quickly, but they can’t get rid of their CO2.
What are examples of Respiratory Acidosis?
1. Low pH
2. High pCO2
3. Variable HCO3- (due to
compensation and chronicity)
4. Is the pH in the 7.35-7.45 window?
What do the following diseases have in common: Hypercortisolism, Adrenal Hyperplasia, Diuretics, etc
= metabolic alkalosis because they all create a basic environment for
the body. In hyperaldosteronism, the kidney is forced to
retain
Na+ at the expense of K+ , but still tries desperately to
regulate the K+ concentration in the body. It is able to reabsorb a
fraction of the K+ , but it costs one H +, causing alkalosis.
What are examples of Metabolic Alkalosis?
1. High pH
2. High HCO3 -
3. Usually High pCO2
4. Is
the pH in the 7.35-7.45 window?
What do the following diseases have in common: Acute Kidney Injury, Diabetes, Salicylate Toxicity, Lactic Acidosis, etc
= metabolic acidosis because each pathology creates an acid that cannot be removed as quickly by the kidneys … so it hangs around for a while.
What are examples of Metabolic Acidosis Example:
1. Low pH
2. Low HCO3-
3. Usually Low pCO2
4. Is the
pH in the 7.35-7.45 window?
What primary and compensatory patterns would you expect in someone with hyperaldosteronism?
Answer:
We would expect to see a primary metabolic alkalosis
with a secondary respiratory acidosis. The mechanism revolves around
the function of aldosterone which retains Na + in exchange for K+
causing hypokalemia. The peritubular capillaries have a H+/K+ exchange
pump which goes at full force to retain as much K+ as possible, but
does so at the cost of a H +. The body is now starving for both H +
and K+ !
What are the common pre-analytical errors and how to minimize them?
1. Blood gases must ALWAYS be collected on ice in a fluoride tube and be assessed within 30 minutes, leukocytes continue to metabolize oxygen after the sample is taken, so rapid assessment is ALWAYS best
2. Electrodes don’t allow for diffusion very easily when there is
protein build-up on the membranes, nor do they function outside of the
standard temperature or barometric pressure that they are supposed to
operate under. However, improper calibration is one of the
most
common sources of error.
3. Probably one of the most common sources of error for the Clarke electrode is improper collection. If the sample can be exposed to room air during collection, transportation, or measurement; be deliberate and cautious with how you handle blood gas samples.
What is the appropriate collection tube, transportation requirement, and time limit to analyze the sample? the lab technician should check for when receiving an ABG sample
Blood gases need to be collected in a fluoride tube (gray top), transported on ice, and assessed within 30 minutes.
A 43-year-old male with a 64 pack-year smoking history presents to
his family physician complaining of being short of breath and for a
cough that “just won’t go away.” He is breathing quickly and a pulse
oximeter shows that his oxygen saturation is 83%. He has been
suffering from emphysema for the last 9 years. What type of acid-base
imbalance would you expect to see in this patient?
A. Respiratory
Alkalosis
B. Respiratory Acidosis
C. Metabolic
Alkalosis
D. Metabolic Acidosis
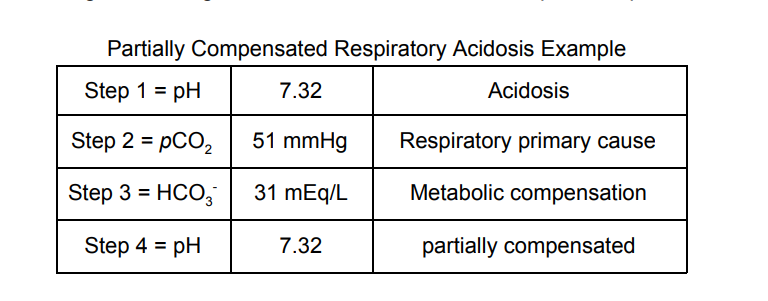
The correct answer is B. Respiratory Acidosis occurs because even
though the patient is likely breathing quickly, he is unable to
efficiently transfer oxygen and carbon dioxide into/out of his blood
because of his pulmonary disease (emphysema). So, if you
were
thinking hyperventilation would cause respiratory alkalosis,
that is a great thought, but each time he breathes, it doesn’t contain
much expired CO2 because of the inefficient gas exchange in his lungs.
Here are values that I would expect in a patient such as this:
2. A 38-year-old female presents to the endocrinologist after
struggling to control her blood pressure for over 5 years. An
abdominal CT scan is ordered and she is diagnosed with an unknown
adrenal mass with primary hyperaldosteronism. On physical exam, the
physician noted that she had bradypnea (slow breathing rate). If the
urinary potassium excretion is high, what would you expect in this
case?
A. Partially compensated metabolic acidosis
B.
Partially compensated metabolic alkalosis
C. Fully compensated
metabolic acidosis
D. Fully compensated metabolic alkalosis
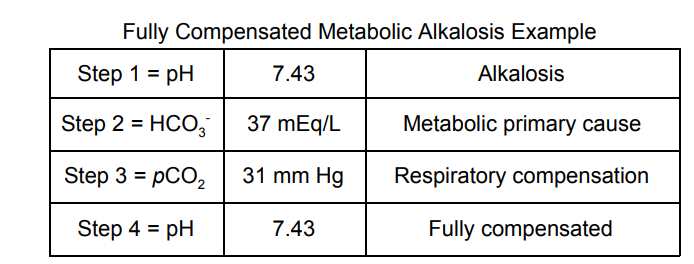
The correct answer is D. Our patient should have a fully compensated
process because she’s been living with it for 5 years. The tumor is
likely hypersecreting aldosterone which will cause the kidneys to
retain Na+ at the expense of K+. One of the main reasons why
this
has an effect on Acid-Base homeostasis is because of a K+/H+ channel
in the distal convoluted tubule of the nephron. Since our body is
short on K + , it’s going to get as much as it can through this
channel. We reclaim a K+, but give up a H+, leaving us in
an
alkalotic state. Here is a panel that I would expect from such
a patient:
A 33-year-old anxious female is feeling overwhelmed about life. She
tells her physician that she can’t seem to accomplish what she wants
to and just thinking about meeting with a doctor about it caused her
to need to sit in her car in the parking lot and “breathe it out.” She
was in obvious distress but after the physician calmed her down, she
apologized for getting “out of control” and drove herself home. What
results would you expect if an ABG were done on this patient?
A.
Partially compensated respiratory acidosis
B. Partially
compensated respiratory alkalosis
C. Uncompensated respiratory
acidosis
D. Uncompensated respiratory alkalosis
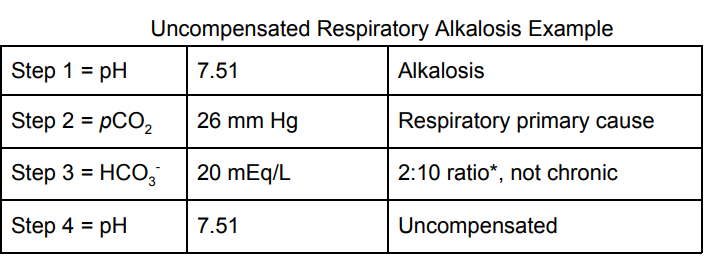
The correct answer is D. This woman is hyperventilating which will
cause her to breathe off her CO2. This will decrease her pCO2 and
thereby, decrease the H + that is produced from carbonic anhydrase.
The circulating HCO3- goes unchecked and causes an alkalotic state.
Because HCO3 - cannot be excreted immediately by the kidney,
the
expected compensatory pattern of metabolic acidosis would not
be seen acutely and would likely take a day or two to develop. Here is
a panel that I would expect from such a patient:
What is the definition of Acidosis?
is any concentration of Hydrogen atoms above the baseline and has a pH lower than 7.35, we see this in vivo as increased pCO2 or decreased HCO3
What is the definition of Alkalosis?
is any concentration of Hydrogen atoms below the baseline and has a pH higher than 7.45, we see this in vivo as decreased pCO2 or increased HCO3
What is the difference between Hypoxia vs Hypoxemia?
Hypoxia is the general term that means we are breathing in less oxygen than we need
Hypoxemia means that we have low levels of oxygen in our blood
For the Henderson-Hasselbalch Equation what ratio of bicarbonate will you get when there is a pH of 7.4?
the ratio of Bicarbonate to Carbonic Acid in the body is 20:1
What is the most important buffer system in the human body?
Bicarbonate Buffer System - H2CO3 and HCO3-
!How is the bicarbonate concentration controlled?!
by the kidneys through metabolic compensation, and usually a Ph change occurs after a few days
!How is the pCO2 concentration controlled?!
by the lungs through respiratory compensation, and can occur immediately
What are the steps for the kidneys to control Acid-base balance?
1.NH3 production and NH4+ secretion in the urine
2.Na-H exchange pumps
3.Conservation of Filtered H2CO3
The tubular lumen is more acidic than the blood
NH3 binds to H+ in the urine filtrate creating NH4+
NH3 can diffuse through plasma membranes, but NH4+ is trapped
What are the steps for gas exchange in the peripheral tissue
1. CO2 carried in RBC
2. HCO3- dissolved in plasma as carbonic acid
3. CO2 dissolved in plasma.
What is the respiratory compensation pattern for: Metabolic acidosis?
Breathe more to reduce the acid to Decrease levels of pCO2 and Increase the levels of HCO3
What is the respiratory compensation pattern for: Metabolic alkalosis ?
Breathe slower to compensate to Increase the levels of pCO2 and Decrease the levels of HCO3
What is partial compensation in breathing?
is when the pH is outside the 7.35-7.45 window, but the pCO2 is indicating one direction (e.g. acidosis) and the HCO3- is indicating another (e.g. alkalosis)
For example, pH of 7.49, pCO2 high, HCO3- very high
What values do you need to know for ABG's
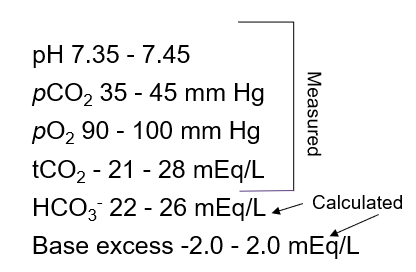
MEMORY TRICK: 35 and 45 have 3, 4, and 5, if you want to extend the chain, just add 2 and 6
A 22-year-old hyper-endurance athlete presents to the physician after running the Leadville 100. He is extremely exhausted, and doesn’t see why it is the case during this race, because it hasn’t been this way in the past. What do you think his laboratory results will indicate?
- Partially compensated metabolic acidosis
- Partially compensated metabolic alkalosis
- Fully compensated metabolic acidosis
- Fully compensated metabolic alkalosis
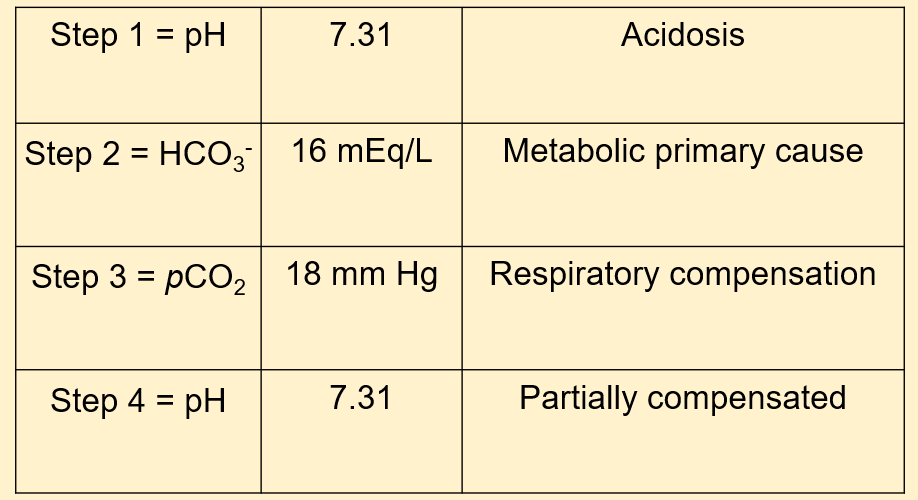
The correct answer is A. Our patient should have a partially compensated process because it’s acutely affecting him. His body is likely unable to respond to the increased demands that he is placing on his muscles in the Leadville 100 race. Lactic acidosis is likely in this scenario. The lactic acid buildup causes a metabolic acidosis and is normally remedied by oxygen and ensuring that there is good blood flow to the affected areas.
A 74-year-old Female is having trouble breathing. She presents at the ER and a chest film is obtained which shows diffuse pulmonary edema and a consolidation in her right lower lobe suggestive of pneumonia. What will her blood gas results likely show?
- Partially compensated respiratory acidosis
- Partially compensated respiratory alkalosis
- Fully compensated respiratory acidosis
- Fully compensated respiratory alkalosis
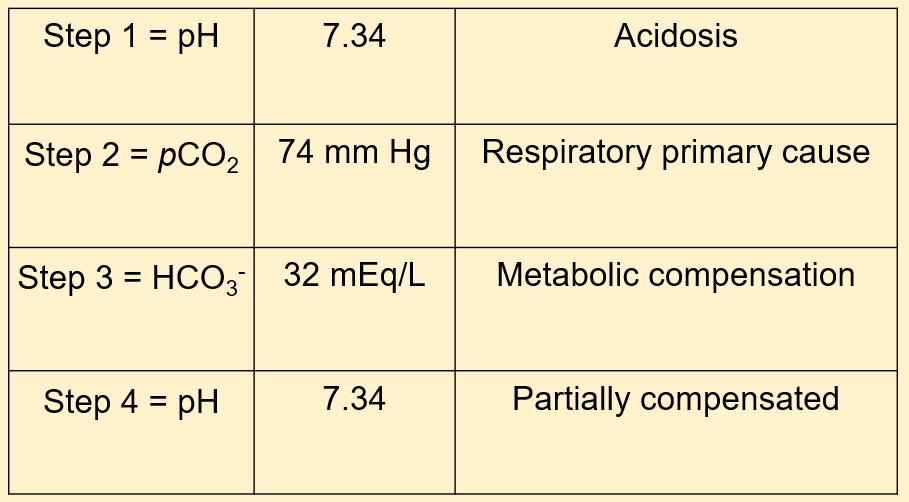
The correct answer is A. Our patient should have a partially compensated respiratory acidosis because it’s acutely affecting her. Her body is trying to respond to decreased oxygen absorption and increased CO2 retention because of the pulmonary edema (fluid on the lungs). She will breathe quicker and her kidneys will retain more HCO3- to balance the respiratory acidosis.
What are the arterial blood gas (ABG) patterns for: Respiratory Alkalosis
pH: up
PaCO2: down
HCO3: Variable
What are the arterial blood gas (ABG) patterns for: Respiratory Acidosis
pH: down
PaCO2: up
HCO3: Variable
What are the arterial blood gas (ABG) patterns for: Metabolic Alkalosis
pH: up
PaCO2: Variable
HCO3: up
What are the arterial blood gas (ABG) patterns for: Metabolic Acidosis
pH: down
PaCO2: Variable
HCO3: down
Given the AGB panel what would it be classified as:
pH 7.31
pCO2 15
HCO3 17
Metabolic acidosis
Given the AGB panel what would it be classified as:
pH 7.22
pCO2 87
HCO3 35
Respiratory acidosis
Given the AGB panel what would it be classified as:
pH 7.03
pCO2 13
HCO3 76
Do not report results
Given the AGB panel what would it be classified as:
pH 7.44
pCO2 13
HCO3 25
Respiratory alkalosis
Given the AGB panel what would it be classified as:
pH 7.56
pCO2 114
HCO3 42
Metabolic alkalosis
Given the AGB panel what would it be classified as:
pH 7.26
pCO2 72
HCO3 17
Mixed acidosis
Where is the standard choice to draw blood from a patient:
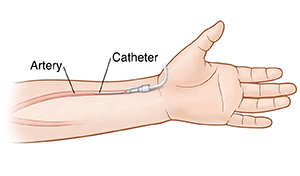
- indwelling arterial catheter in the radial artery: Note: these are places of error.
- Test is performed on Heparinized Whole Blood
- Collection is in a glass syringe with lyophilized heparin
- Specimen is stable on ICE for 1 hour and at RT for 30 minutes
What are ranges of samples that are exposed to air?
pH > 7.45 and a pCO2 < 35
What are sources for preanalytical error?
- wrong patient drawn
- inappropriate draw technique
- failure to follow protocol for drawing specimen
- introducing air into the syringe
- exposing the specimen to air
- delays in transport
- not keeping sample cold during transport
- failure to check patient demographics
- failure to mix sample properly
- failure to remove all air bubbles
- failure to remove fibrin clots
What are sources for analytical error?
- Creating a short sample
- failure to perform quality control
- failure to perform maintenance
- failure to perform calibration
What are sources for postanalytical error?
- reporting wrong results to the clinician
- transcription mistakes
- failure to interpret instrument flags or warning something may be wrong
How often is calibration required for a Clark electrode?
! Two points of calibration are required every eight hours and one point calibration at least every four hours. Because of electrode drift slow up ward or down ward drift.
What is potassium shift?
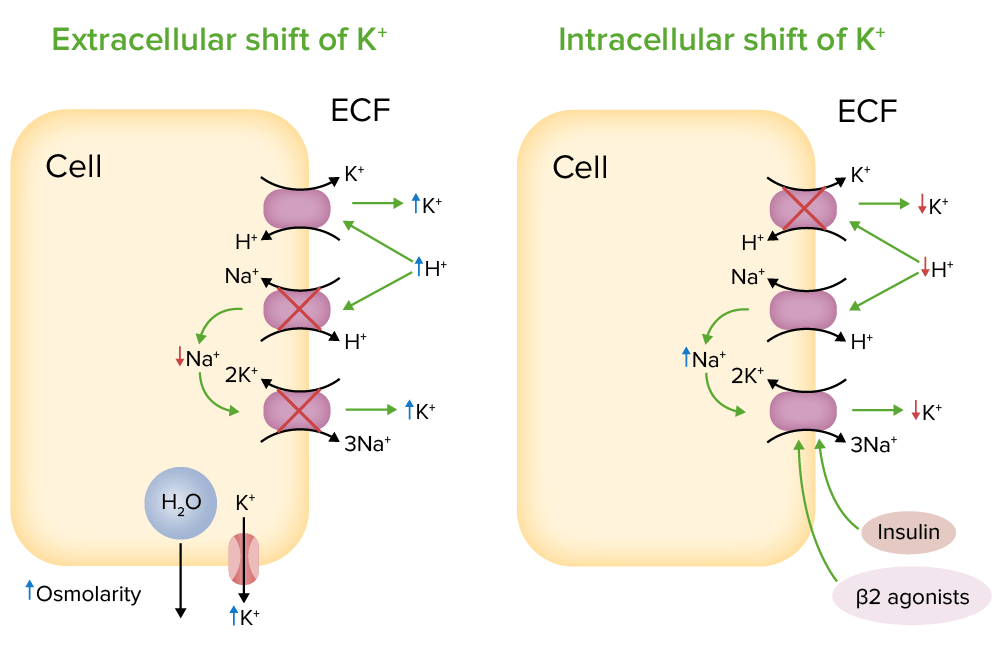
An increase in extracellular H+ ions will drive K+ out. The cell is nice enough to offer to take some of the burden of harboring the extra H+, but it does so in exchange for K+ to keep electrical neutrality
What is Chloride shift?
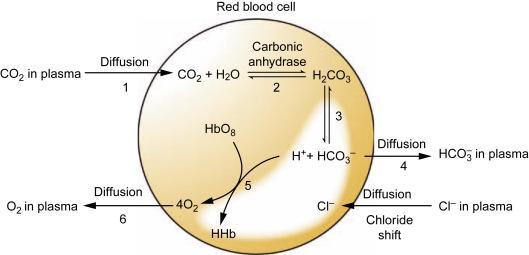
The high H+ concentration in the tissues draws the HCO- out of the cells and sucks the Cl- intracellularly
The lower H+ concentration in the lungs pulls the HCO- back into the cells and pumps the Cl- out
What acid base abnormality does someone have who is hyperventilating?
Primary Respiratory Alkalosis - Cause: Blow off CO2
By product Response: Compensatory Metabolic Acidosis - Excrete more HCO3- in the urine
What acid-base abnormality occurs in someone with inefficient gas exchange?
Primary Respiratory Acidosis - Cause: Can’t get rid of enough CO2
Response: Compensatory Metabolic Alkalosis - Retain more HCO3- in the kidneys
What acid-base abnormality occurs in someone with Ethanol Poisoning?
Primary Metabolic Acidosis - Cause: Ethanol causes a lactic acidosis
Response: Compensatory Respiratory Alkalosis - Breathe quickly to release extra H+
What acid-base abnormality occurs in someone who is severely vomiting?
Primary Metabolic Alkalosis - Cause: Loss of Hydrochloric Acid in vomitus
Response: Compensatory Respiratory Acidosis - Breathe slowly to retain H+
Given the AGB panel what would it be classified as:
pH 7.51
pCO2 79
HCO3 53
Metabolic alkalosis
Given the AGB panel what would it be classified as:
pH 7.39
pCO2 53
HCO3 51
Respiratory acidosis
Given the AGB panel what would it be classified as:
pH 7.29
pCO2 91
HCO3 16
Mixed acidosis
Given the AGB panel what would it be classified as:
pH 7.49
pCO2 11
HCO3 22
Respiratory alkalosis
Given the AGB panel what would it be classified as:
pH 7.56
pCO2 21
HCO3 62
Mixed alkalosis
Given the AGB panel what would it be classified as:
pH 7.38
pCO2 72
HCO3 56
Respiratory acidosis
If the PH is above or bellow 7.4 what does it mean?
Bellow 7.4= acidosis
above 7.4= alkalosis
If the PCO2 level is bellow 35 and above 45 what can you conclude?
then the cause is some sort of respiratory process
If the HCO3 is below 22 or above 28 what can you conclude?
then you should think of metabolic causes.