Microbial Metabolism Chapter 5
reaction that PRODUCES energy by causing a molecule to LOSE electrons
OXIDATION REACTION
reaction that REQUIRES energy in causing a molecule to GAIN electrons
REDUCTION REACTION
electrons that are lost in the oxidation reaction are the same electrons that are gained in the reduction reaction. these two reactions are collectively called
REDOX ( REDuction/ OXidation) REACTION
1. stores chemical energy released by catabolic reactions
2. provides energy for anabolic reactions
FUNCTION OF ATP
ADDING phosphate and STORING energy
PHOSPHORYLATION
REMOVING phosphate and RELEASING energy
DE-PHOSPHORYLATION
inhibition that occurs when the
-end product of a metabolic pathway inhibits the enzyme activity near the start of the pathway
FEEDBACK INHIBITION
what is the FINAL electron acceptor (END PRODUCT) in AEROBIC respiration
OXYGEN (O2)
what is the FINAL electron acceptor (END PRODUCT) in ANAEROBIC respiration
any INORGANIC molecule other than OXYGEN (O2)
the ONLY enzyme that is NOT a protein
RIBOENZYME
cut and splice RNA in EUKARYOTIC cells
what is the FUNCTION OF A RIBOENZYME
enzyme inhibitors can be
- COMPETITIVE- compete directly for the active site
- NON-competitive- compete INdirectly for the alosteric site- ALLOSTERIC INHIBITION
a substance that binds to the enzyme and induces the enzyme's inactive form- NON-competitive inhibition
ALLOSTERIC INHIBITION
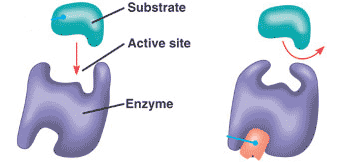
what causes an active site to CHANGE SHAPE preventing the substrate from fitting
ALLOSTERIC SITE
the process by which living organisms AEROBICALLY harvest energy from food
CELLULAR RESPIRATION
what is glycolysis
the SPLITTING of sugar
one glucose molecule produce TWO _________ and TWO __________
ATP & NADH
the end product of glycolysis
- PYRUVIC ACID (pyruvate)
- ATP
- NADH
how many molecules of ATP are invested in glycolsis
TWO
in fermentation the final electron acceptor is
ORGANIC MOLECULE
PARTIAL oxidation of glucose (glucose is partially broken down) in the absence of oxygen
FERMENTATION
does FERMENTATION produce any ATP? if so.....how many
YES fermentation produces TWO ATP
normal byproduct of muscle metabolism, that can irritate muscles and cause discomfort and sorenes (cramps)
LACTIC ACID
small port in an enzyme where substrate molecules bind and undergo a chemical reaction
ACTIVE SITE
what molecule binds at the active site
SUBSTRATE MOLECULES
a molecule being acted on by an enzyme to make another product
SUBSTRATE
a NON-protein component (other than the substrate) whose presence is essential for the activity of an enzyme
CO-FACTOR
what factors affect enzymes
- TEMPERATURE (LOW-HIGH)
- pH (7 optimal)
- SUBSTRATE CONCENTRATION (goes high and plateaus)
- SATURATION
BREAKING DOWN of more complex organic molecules into simpler substance and RELEASE energy
CATABOLISM (think catastrophe)
CATABOLIC REACTION
BUILDING UP of simplier substances to form more complex molecules and REQUIRE energy
ANABOLISM
ANABOLIC REACTION
the two major type of glucose catabolism are ________________ in which glucose is COMPLETELY broken down and________________ in which glucose is PARTIALLY broken down
RESPIRATION
FERMENTATION
the most commonly used carbohydrate
GLUCOSE
most of a cells energy is produced from
OXIDATION OF CARBOHYDRATES
the sum of all chemical reactions
METABOLISM
NON-active protein portion of an enzyme
APOenzyme
cells extract energy and store is as
ATP
proteins produced by living cells that catalyze chemical reactions by lowering activation energy
ENZYME
- catalyst
- speed up chemical reactions
- LOWER the activation energy of the chemical reaction
- specific to ONE reaction
- act on a specific substrate
FUNCTIONS OF ENZYMES
- a nonprotein compound that is necessary for the functioning of an enzyme
- They bind to the active site of the enzyme and participate in catalysis but are not considered substrates of the reaction
- Example NAD
Co-Enzyme
co-factors are composed mainly of
METAL IONS
an enzyme complete with both its APOenzyme and COenzyme components
HOLOENZYME
MAX number of substrate molecules an enzyme can convert to a product each second
TURNOVER NUMBER
10,000 - several millin molecular weight is the standard size of
ENZYME
enzyme name usually end in
-ase
each time a substance is oxidized another is simultaneously reduced
REDOX
NAD+ is reduced or oxidized
OXIDIZED FORM
NADH is reduced or oxidized
REDUCED (gained Hydrogen)
ADDING a P to a molecule is called
PHOSPHORYLATION
the most usable form of energy
ATP
most common pathway for the oxidation of glucose
GLYCOLYSIS
what is glycolsis
the breaking down of pyruvic acid producing small amounts of ATP and NADH that occurs in the cytoplas m
does fermentation require oxygen
NO
what are the two types of fermentation
1. lactic acid fermentation
2. alcohol fermentation
what determines whether cellular respiration is aerobic and anaerobic
the presence of oxygen
how much ATP does ANAerobic respiration produce
2 ATP
how much ATP can AERobic respiration produce
36-38 ATP