Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Microbial Metabolism Chapter 5
front 1 reaction that PRODUCES energy by causing a molecule to LOSE electrons | back 1 OXIDATION REACTION |
front 2 reaction that REQUIRES energy in causing a molecule to GAIN electrons | back 2 REDUCTION REACTION |
front 3 electrons that are lost in the oxidation reaction are the same electrons that are gained in the reduction reaction. these two reactions are collectively called | back 3 REDOX ( REDuction/ OXidation) REACTION |
front 4 1. stores chemical energy released by catabolic reactions 2. provides energy for anabolic reactions | back 4 FUNCTION OF ATP |
front 5 ADDING phosphate and STORING energy | back 5 PHOSPHORYLATION |
front 6 REMOVING phosphate and RELEASING energy | back 6 DE-PHOSPHORYLATION |
front 7 inhibition that occurs when the -end product of a metabolic pathway inhibits the enzyme activity near the start of the pathway | back 7 FEEDBACK INHIBITION |
front 8 what is the FINAL electron acceptor (END PRODUCT) in AEROBIC respiration | back 8 OXYGEN (O2) |
front 9 what is the FINAL electron acceptor (END PRODUCT) in ANAEROBIC respiration | back 9 any INORGANIC molecule other than OXYGEN (O2) |
front 10 the ONLY enzyme that is NOT a protein | back 10 RIBOENZYME |
front 11 cut and splice RNA in EUKARYOTIC cells | back 11 what is the FUNCTION OF A RIBOENZYME |
front 12 enzyme inhibitors can be | back 12
|
front 13 a substance that binds to the enzyme and induces the enzyme's inactive form- NON-competitive inhibition | back 13 ALLOSTERIC INHIBITION |
front 14 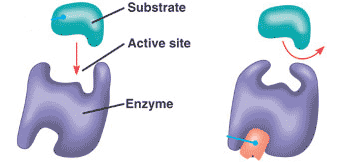 what causes an active site to CHANGE SHAPE preventing the substrate from fitting | back 14 ALLOSTERIC SITE |
front 15 the process by which living organisms AEROBICALLY harvest energy from food | back 15 CELLULAR RESPIRATION |
front 16 what is glycolysis | back 16 the SPLITTING of sugar |
front 17 one glucose molecule produce TWO _________ and TWO __________ | back 17 ATP & NADH |
front 18 the end product of glycolysis | back 18
|
front 19 how many molecules of ATP are invested in glycolsis | back 19 TWO |
front 20 in fermentation the final electron acceptor is | back 20 ORGANIC MOLECULE |
front 21 PARTIAL oxidation of glucose (glucose is partially broken down) in the absence of oxygen | back 21 FERMENTATION |
front 22 does FERMENTATION produce any ATP? if so.....how many | back 22 YES fermentation produces TWO ATP |
front 23 normal byproduct of muscle metabolism, that can irritate muscles and cause discomfort and sorenes (cramps) | back 23 LACTIC ACID |
front 24 small port in an enzyme where substrate molecules bind and undergo a chemical reaction | back 24 ACTIVE SITE |
front 25 what molecule binds at the active site | back 25 SUBSTRATE MOLECULES |
front 26 a molecule being acted on by an enzyme to make another product | back 26 SUBSTRATE |
front 27 a NON-protein component (other than the substrate) whose presence is essential for the activity of an enzyme | back 27 CO-FACTOR |
front 28 what factors affect enzymes | back 28
|
front 29 BREAKING DOWN of more complex organic molecules into simpler substance and RELEASE energy | back 29 CATABOLISM (think catastrophe) CATABOLIC REACTION |
front 30 BUILDING UP of simplier substances to form more complex molecules and REQUIRE energy | back 30 ANABOLISM ANABOLIC REACTION |
front 31 the two major type of glucose catabolism are ________________ in which glucose is COMPLETELY broken down and________________ in which glucose is PARTIALLY broken down | back 31 RESPIRATION FERMENTATION |
front 32 the most commonly used carbohydrate | back 32 GLUCOSE |
front 33 most of a cells energy is produced from | back 33 OXIDATION OF CARBOHYDRATES |
front 34 the sum of all chemical reactions | back 34 METABOLISM |
front 35 NON-active protein portion of an enzyme | back 35 APOenzyme |
front 36 cells extract energy and store is as | back 36 ATP |
front 37 proteins produced by living cells that catalyze chemical reactions by lowering activation energy | back 37 ENZYME |
front 38
| back 38 FUNCTIONS OF ENZYMES |
front 39
| back 39 Co-Enzyme |
front 40 co-factors are composed mainly of | back 40 METAL IONS |
front 41 an enzyme complete with both its APOenzyme and COenzyme components | back 41 HOLOENZYME |
front 42 MAX number of substrate molecules an enzyme can convert to a product each second | back 42 TURNOVER NUMBER |
front 43 10,000 - several millin molecular weight is the standard size of | back 43 ENZYME |
front 44 enzyme name usually end in | back 44 -ase |
front 45 each time a substance is oxidized another is simultaneously reduced | back 45 REDOX |
front 46 NAD+ is reduced or oxidized | back 46 OXIDIZED FORM |
front 47 NADH is reduced or oxidized | back 47 REDUCED (gained Hydrogen) |
front 48 ADDING a P to a molecule is called | back 48 PHOSPHORYLATION |
front 49 the most usable form of energy | back 49 ATP |
front 50 most common pathway for the oxidation of glucose | back 50 GLYCOLYSIS |
front 51 what is glycolsis | back 51 the breaking down of pyruvic acid producing small amounts of ATP and NADH that occurs in the cytoplas m |
front 52 does fermentation require oxygen | back 52 NO |
front 53 what are the two types of fermentation | back 53 1. lactic acid fermentation 2. alcohol fermentation |
front 54 what determines whether cellular respiration is aerobic and anaerobic | back 54 the presence of oxygen |
front 55 how much ATP does ANAerobic respiration produce | back 55 2 ATP |
front 56 how much ATP can AERobic respiration produce | back 56 36-38 ATP |