Molecular Toxicology Exam 2 Review
What is oxidative stress
Imbalance of pro-oxidizing compounds and antioxidants
pivotal mechanism for many xenobiotics
What are the two mechanisms for which oxidative stress occur
1) Increased ROS ---> oxidative stress
2) Depletion of antioxidant pool ---> oxidative stress
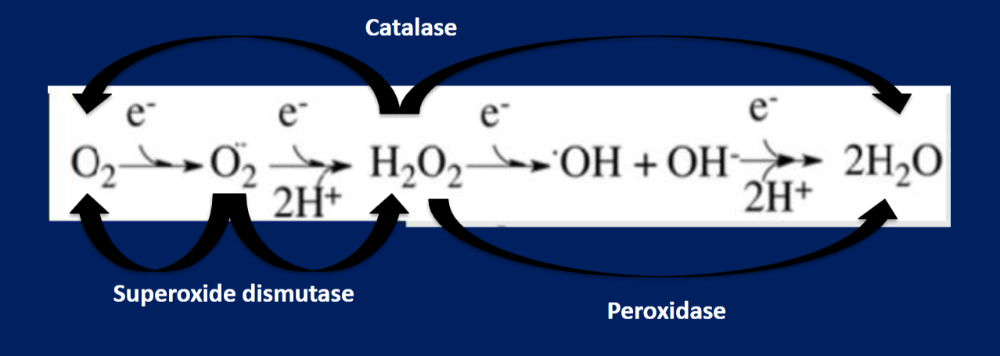
How is molecular oxygen reduced (draw the stepwise mechanism)
Label what antioxidant works on what
How much ROS usually escapes the mitochondria
Usually less than 1%
What do toxins do regarding oxidative stress?
Increase ROS or decrease antioxidant pool
What produces extracellular ROS and give an example
Generated by phagocytes or lymphocytes
Example: NADPH oxidase - dormant, membrane bound protein which is activated by surface cell receptors and can produce ROS
What are 4 important things antioxidants do
1) Provide alternative substrate for ROS: gives it something else to bind to
- Most antioxidants have a lot of thiol groups (sulfur groups) to bind ROS
2) Keep cellular thiol in its reduced form (not oxidized)
3) Prevent/repair oxidation of cellular components
4) Sequester redox active metals
- Metallothionine: has lots of sulfurs to neutralize ROS with binding
Are antioxidants enzymatic or non-enzymatic?
They can be either
How does antioxidant potential work (give an example comparison)
It differs between organs
ex: Liver/kidney have high antioxidant potential since they the natural detoxifiers of the body. They are the first to be exposed to xenobiotics and poisons unlike your CNS/cardiac which has a low antioxidant potential
Answer these next few questions:
What antioxidants act on hydrogen peroxide?
What antioxidant acts on the superoxide radical?
What antioxidant acts on the hydroxyl radical?
1) Catalase and peroxidase
2) Superoxide dismutase (SoD)
3) it doesn't have one; it is scavenged by endogenous compounds to be bound up and excreted
Define superoxide dismutase
- SoD
-Enzymatic
- most important antioxidant mutant
-
What three genes code for superoxide dismutase?
SoD1 = cytosolic
SoD2 = mitochondrial
Sod3 = extracellular
What makes SoD critical?
Every aerobic organism processes SoD
Mutations of SoD linked to lou gehrigs disease
Where is catalase found
Peroxisomes
Where is gultathion peroxidase found
cytosol
Name off what you know about Glutathione (GSH)
tripeptide of glutamide, cystine, and glycine
by itself it is the most important non-enzymatic antioxidant
it has a large number of cystines
GSH is found in mM concentration in many tissues
Has three pools within cells: Nuclear, mitochondrial, and cystolic
What are glutathione's two mechanisms?
1) Non-enzymatic scavenging where GSH turns into GSSH
2) Enzymatic where thiol reductase reduces disulfide bonds in oxidized proteins and glutathione peroxidase is found
What are the major ways glutathione pools are depleted and give one example of an effect
1) chronic exposure to pro-oxidants
2) Depletion of a necessary source like nutrition ie. fasting/dieting (toxicity may occur due to low glutathione
ex. GGPD Deficency: most common pharmacogenic polymorphism
What happens with moderate increases of intracellular Ca
Activates Ca dependent caspases (low concentration of xenobiotics)
What happens with high increases of intracellular Ca
Release from intracellular Ca stores
4 important things that are affected by high Ca and what happens?
1) Proteases and Calpain activation
- Activity is regulated by Ca concen. + calpain inhibitor
- Can facilitate apoptosis by assisting caspases
2) Endonucleases
- Role is to cleave DNA
- Result in single/double bond breaks resulting in apoptosis
- ie. acetominophen
3) Lipases and membranes
- Phospholipase A2 is activated which causes Arachidonic Acid production
- AA activates mpt which causes a large pore to form in the mitochondria
4) Mitochondria
-3 important parts are Ca transport pumps, proton gradient, and ATP production
-CC in mitochondria can move out into extracellular space after pore is produced facilitating fast apoptosis already occuring
What is cell death caused by
oxidative stress, covalent binding, or mitochondrial injury
how is necrosis v apoptosis determined
extent of cellular damage or cellular energy status
Necrosis information
-cells swell to rupture
- cells lose ability to osmoregulate
5 steps of necrosis activation
1) oxi stress, mito damage, or Ca influx
2) Changes in plasma membrane permeability (loss of ion control)
3) cytoskeleton breaks down
4) ATP is depleted (pumps break, loses ability to maintain things like Na, Ca)
5) swelling and burst
Progression of necrosis
- propagation across cells
- does not require toxin to be present anymore
- spilled cellular contents cause ROS to be produced in extracellular space where Ca is very present, causes dormant lipases and proteases to be activated affecting neighboring cells
Apoptosis information
- cells packaged for disposal
- cell membrane does no lyse
Steps of apoptosis
1) initiating event ( cell signaling event etc)
2) Activation of caspases (one caspase is an amplifier used to activate the rest of the caspases which break down cellular components)
3)DNA condenses and is cleaved into 180bp fragments (nucleus is very apparent, activated by nucleases)
4)More caspases break down cell proteins (leads to rapid collapse in cell functionality)
5) Cell body shrinks and produces apoptotic bodies
6) apop bodies are phagocytized by macrophages until the cell is gone
What are death receptors and their three domains (draw death receptor)
large group of cell membrane receptors that activate apoptosis
3 domains
- extracellular domain ( cystine rich (sulfurs))
- membrane signalling domain
-Intracellular death sequence domain
How do death receptors work? give an example
- DR's trimerize through cellular interactions
- trimerized complex can bind with specific DR's to activate
- Recruitment of accessory proteins results in disc formation
- DISC activates caspases
Ex. FAS: DR typically stored intracellularly normally binds to fasL for activation, can bind to itself to initiate apoptosis
What is cytochrome C
- very large molecule found inside mitochondrial membrane, has to be transported out of the mitochondria
- can directly activate apoptotic caspases
- apoptotic signaling proteins or oxidative damage can open pore and allow cyt C out into cytosol
What are the regulators of Cytochrome C
Bcl-2: produced as an anti-apoptotic factors, forms dimers and prevents mpt from opening
BAX protein: upregulated by DR's, causes mpt pore opening and release of cyt-c
Bid: Activated by caspases to form tbid and migrates to nucleus, increases ROS production in mitochondria, damages cell membrane, promotes cyt-c leakage
What is mitogenesis
Repairing of lost cells for low-level apoptosis
What is cell proliferation
increased mitotic activity in response to toxic insult; used to restore tissue to normal mass and function
Toxins can inhibit or promote this process leading to no cell regeneration or tumor growth
what is a direct mitogen
Compounds that directly push cells into mitotic cycle w/out previous damage
leads to tumor growth
ex.lead: Pb2+ activates Ca2+ sensitive proteins and pkc phosphorylates proteins which induce DNA synthesis
What is an antimitogen and give an example
Inhibit cell cycle progression and blocks mitosis
Taxol: anticancer drug that effects formation of mitotic spindle apparatus
- it does not allow the spindle to disassemble , preventing mitosis and eventually causing apoptosis