CH 2
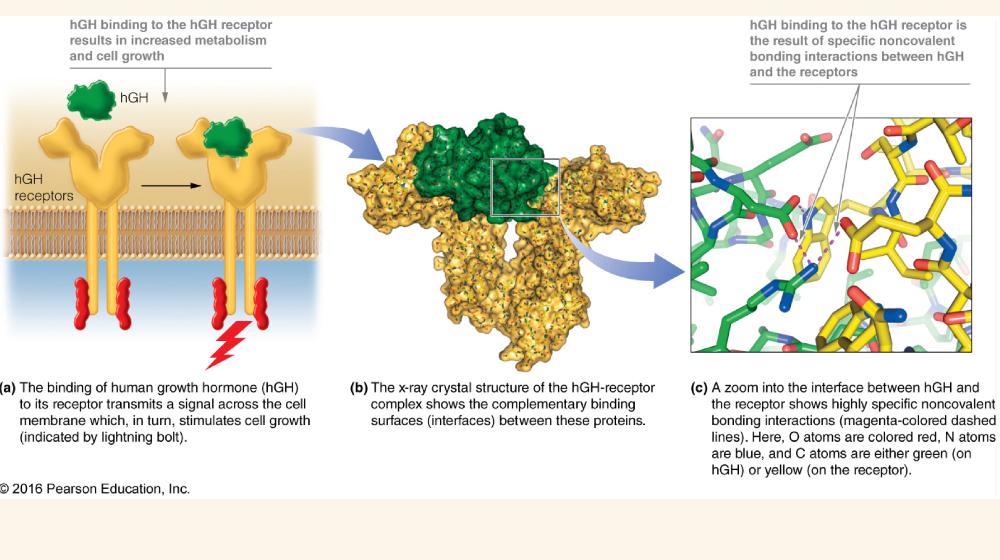
Fig 2.1 Noncovalent bonding interactions between human growth hormone and its cellular receptor
DNA strains are maintained by
covalent bonds
Protein made up of amino acids linked by
Covalent peptide bonds
Types of noncovalent interactions
- Charge- charge (Ionic Bonds)
- Charge-dipole
- Dipole-Dipole
- Dipole-induced dipole
- Dispersion(van der Waals)
- Hydrogen Bond
Proteins carry more positive charge at what?
lower pH levels
Extreme ph, leading to protein denaturation due to loss of charge and mutual repulsion are called what?
Salt Bridges
law describes the force between charged particles, which is reduced in biological environments due to the screening effect of water and other molecules.
Coulombs law
Q: How do you calculate the pI of an amino acid like aspartic acid?
Average the two pKa values around the neutral (zwitterionic) form.
For aspartic acid:
\text{pI} = \frac{2.1 + 3.9}{2} = 3.0
What happens when pH < pI?
A: The molecule carries a net positive charge.
Q: What happens when pH > pI?
A: The molecule carries a net negative charge.
Q: What happens when pH = pI?
A: The molecule is neutral overall (zwitterion).
Q: What changes the pI of a protein?
: The types of ionizable groups on its surface:
- More acidic groups → lower pI
- More basic groups → higher pI
What is a polyelectrolyte?
A: A molecule with many ionizable (charged) groups.
What’s the difference between strong and weak polyelectrolytes?
- Strong: Stay charged over wide pH ranges (e.g., DNA)
- Weak: Gradually lose/gain protons as pH changes (e.g., polylysine)
Why is understanding pI important in biochemistry?
It affects protein solubility, structure, function, and is used in techniques like electrophoresis and isoelectric focusing.
Where do most biochemical reactions occur?
A: In aqueous (water-based) environments—except inside hydrophobic membranes.
Q: What are Brønsted-Lowry acids and bases?
A: Acids are proton donors; bases are proton acceptors.
Q: What is the difference between strong and weak acids?
A: Strong acids fully dissociate; weak acids partially dissociate in water.
Q: What is the ion product of water (Kw) at 25°C?
K_w = [H^+][OH^-] = 1 \times 10^{-14}
Q: What is pH?
\text{pH} = -\log[H^+]
Lower pH = more acidic; higher pH = more basic.
What is the physiological pH range for most biochemical reactions?
A: Between 6.5 and 8.0
Q: How does pH affect biomolecules?
A: It changes their protonation state and overall charge, affecting function and interactions.
Q: What is Ka and pKa?
- Ka = acid dissociation constant
- pKa = –log(Ka) → lower pKa = stronger acid
What is the Henderson–Hasselbalch Equation?
A:
\text{pH} = \text{pKa} + \log\left(\frac{[\text{A}^-]}{[\text{HA}]}\right)
When is pH = pKa in a titration?
At the midpoint, where [HA] = [A⁻]
Q: What is a buffer solution?
A: A mixture of weak acid and its conjugate base that resists pH changes.
Q: Why is buffering important in biology?
A: It maintains stable pH for enzymes and cell function, especially in blood and cells.
Q: What happens if blood pH falls below 7.2?
A: It can lead to acidemia, a medical emergency linked to diseases like diabetes.
Q: How do you calculate pI for a molecule with two ionizable groups?
\text{pI} = \frac{\text{pKa}_1 + \text{pKa}_2}{2}
What is a zwitterion?
A: A molecule with both positive and negative charges, but net charge = 0.
Q: What are ampholytes?
A: Molecules with both acidic and basic groups that can act as buffers in different pH ranges.
Q: What happens to a molecule’s charge as pH increases?
A: It becomes more negatively charged (deprotonated).
Q: What happens to a molecule’s charge as pH decreases?
A: It becomes more positively charged (protonated).
Q: Name two major biological buffer systems
- Phosphate buffer (intracellular, pKa ≈ 6.86)
- Bicarbonate buffer (blood, apparent pKa ≈ 6.3)