Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
CH 2
front 1 Fig 2.1 Noncovalent bonding interactions between human growth hormone and its cellular receptor | back 1 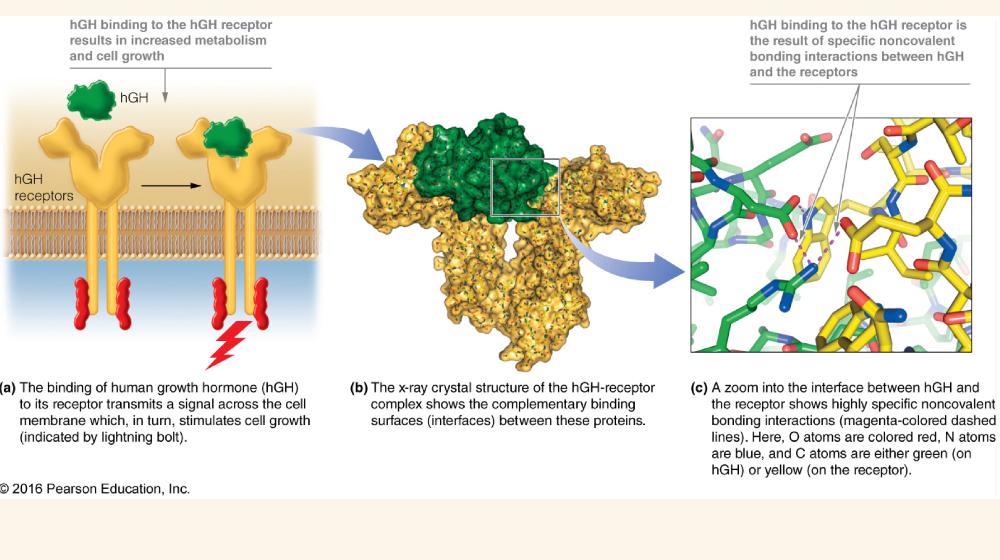 |
front 2 DNA strains are maintained by | back 2 covalent bonds |
front 3 Protein made up of amino acids linked by | back 3 Covalent peptide bonds |
front 4 Types of noncovalent interactions | back 4 - Charge- charge (Ionic Bonds) - Charge-dipole - Dipole-Dipole - Dipole-induced dipole - Dispersion(van der Waals) - Hydrogen Bond |
front 5 Proteins carry more positive charge at what? | back 5 lower pH levels |
front 6 Extreme ph, leading to protein denaturation due to loss of charge and mutual repulsion are called what? | back 6 Salt Bridges |
front 7 law describes the force between charged particles, which is reduced in biological environments due to the screening effect of water and other molecules. | back 7 Coulombs law |
front 8 Q: How do you calculate the pI of an amino acid like aspartic acid? | back 8 Average the two pKa values around the neutral (zwitterionic) form. For aspartic acid: \text{pI} = \frac{2.1 + 3.9}{2} = 3.0 |
front 9 What happens when pH < pI? | back 9 A: The molecule carries a net positive charge. |
front 10 Q: What happens when pH > pI? | back 10 A: The molecule carries a net negative charge. |
front 11 Q: What happens when pH = pI? | back 11 A: The molecule is neutral overall (zwitterion). |
front 12 Q: What changes the pI of a protein? | back 12 : The types of ionizable groups on its surface:
|
front 13 What is a polyelectrolyte? | back 13 A: A molecule with many ionizable (charged) groups. |
front 14 What’s the difference between strong and weak polyelectrolytes? | back 14
|
front 15 Why is understanding pI important in biochemistry? | back 15 It affects protein solubility, structure, function, and is used in techniques like electrophoresis and isoelectric focusing. |
front 16 Where do most biochemical reactions occur? | back 16 A: In aqueous (water-based) environments—except inside hydrophobic membranes. |
front 17 Q: What are Brønsted-Lowry acids and bases? | back 17 A: Acids are proton donors; bases are proton acceptors. |
front 18 Q: What is the difference between strong and weak acids? | back 18 A: Strong acids fully dissociate; weak acids partially dissociate in water. |
front 19 Q: What is the ion product of water (Kw) at 25°C? | back 19 K_w = [H^+][OH^-] = 1 \times 10^{-14} |
front 20 Q: What is pH? | back 20 \text{pH} = -\log[H^+] Lower pH = more acidic; higher pH = more basic. |
front 21 What is the physiological pH range for most biochemical reactions? | back 21 A: Between 6.5 and 8.0 |
front 22 Q: How does pH affect biomolecules? | back 22 A: It changes their protonation state and overall charge, affecting function and interactions. |
front 23 Q: What is Ka and pKa? | back 23
|
front 24 What is the Henderson–Hasselbalch Equation? | back 24 A: \text{pH} = \text{pKa} + \log\left(\frac{[\text{A}^-]}{[\text{HA}]}\right) |
front 25 When is pH = pKa in a titration? | back 25 At the midpoint, where [HA] = [A⁻] |
front 26 Q: What is a buffer solution? | back 26 A: A mixture of weak acid and its conjugate base that resists pH changes. |
front 27 Q: Why is buffering important in biology? | back 27 A: It maintains stable pH for enzymes and cell function, especially in blood and cells. |
front 28 Q: What happens if blood pH falls below 7.2? | back 28 A: It can lead to acidemia, a medical emergency linked to diseases like diabetes. |
front 29 Q: How do you calculate pI for a molecule with two ionizable groups? | back 29 \text{pI} = \frac{\text{pKa}_1 + \text{pKa}_2}{2} |
front 30 What is a zwitterion? | back 30 A: A molecule with both positive and negative charges, but net charge = 0. |
front 31 Q: What are ampholytes? | back 31 A: Molecules with both acidic and basic groups that can act as buffers in different pH ranges. |
front 32 Q: What happens to a molecule’s charge as pH increases? | back 32 A: It becomes more negatively charged (deprotonated). |
front 33 Q: What happens to a molecule’s charge as pH decreases? | back 33 A: It becomes more positively charged (protonated). |
front 34 Q: Name two major biological buffer systems | back 34
|