Review for Orgo 2 Exam (that i keep forgetting)
hydrates
gem diol
reaction Reversible to an aldehyde/ketone
Relatively stable by the electron-withdrawing groups
Hemiacetal
OH and OR
Can occur under acidic, basic, neutral conditions
Reversible like hydrates
Intramolecular Hemiacetal formation
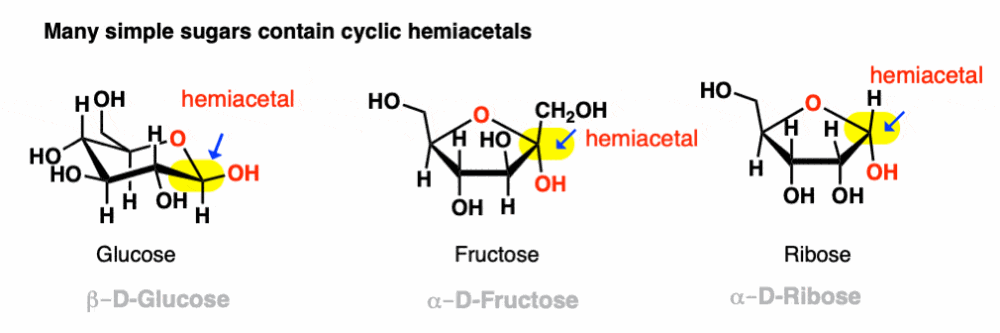
Alcohol + Aldehyde will most likely form a cyclic hemiacetal
Cyclic hemiacetal can revert to acyclic alcohol by NaBH4
PADE P(protonation) A(addition) D(deprotonation) E(elimination)
Reaction of Acetal
Are locked and not reversible so no reaction can take place after an acetal formation
Undergo one reaction which is hydrolysis (H3O+
Acetal Protection Group
A Grignard reagent cannot be used with aldehydes or ketones, as it will react with them immediately.
We can use ethylene glycol or Ch3OH H+ to protect
Thioacetals
Raney Ni (reduces to alkanes) as well as the wolff-kisher and clemmensen
Degree of Unsaturation
Formula: 2C+2+N-H-X/2
Hydrocarbon(no rings/double bonds) formula
Key Pattern formula: # Hydrogens = 2 x (# of Carbons) + 2
Pi bond reduce the hydrogen count by 2
IR spectroscopy
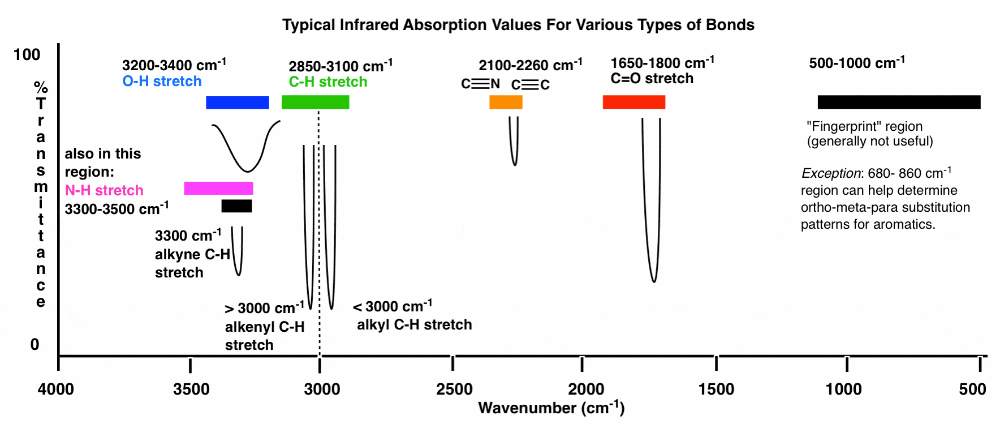
3600 – 2700 cm-1X-H (single bonds to hydrogen)
2700 – 1900 cm-1 X≡X (triple bonds)
1900 – 1500 cm-1 X=X (double bonds)
1500 – 500 cm -1X–X (single bonds)
3000 border between alkene(above) and alkane(below)
dont look for degrees of unsat if there isnt any
More important notes of IR
3400-3200 (OH appear)
1850 -1630 (C=O) [like swords]
3200 Amines and Amides Appear
- Primary Amine: 2 stretched-out small peaks (like balls)
- Secondary Amine: 1 long stretch peak
- Primary Amide: 2 peaks a little more spaced out (like boobs
- Secondary Amide: 1 long a little open peak further down
(rare) Triple bond region around 2050-2250 cm-1
More info
Aldehydes(1740-1690)
Ketones (1750-1680)
Esters (1750 -1735)
Carboxylic acids (1780 -1710)
Amide (1690-1630)
Anhydrides (1830-1800) (1775-1740)
Monoasscharides
Glucose, Fructose
Disaccharide
Sucrose, Maltose, Lactose
Epimers
When two diastereomers only different from 1 carbon
Fisher Projects for Glycosides
OH on the right (goes down)
OH on the left (goes up)
5 carbon OH on the right is (D)
5 carbon OH on the left is (L)
5 and 6 carbon bonds rotates during glycoside formation and if OH is down, it does not become up. If up down
Glycosides formation
Carbon 1: Up = Beta (more predominant) (more likely seen)
Carbon 1: Down = Alpha
Reducing Sugar for Carbohydrates
Hemiacetal and Aldehydes/ketones
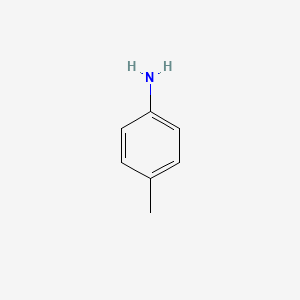
p - Toluidine
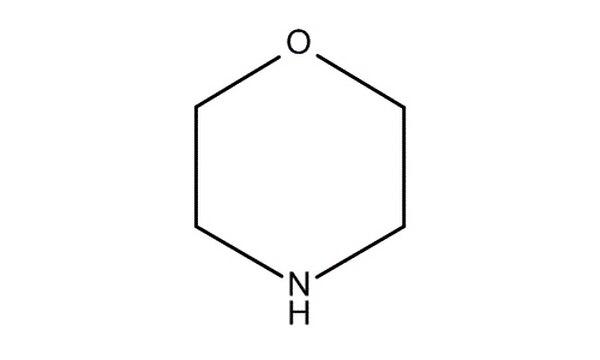
Morpholine
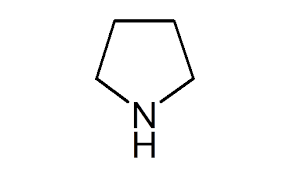
Pyrrolidine
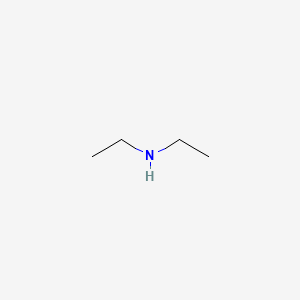
Diethylamine
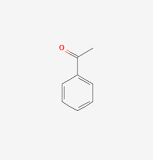
Acetophenone
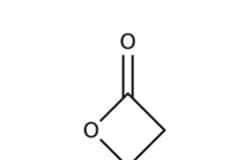
Lactone
Ranking Nucleophiles
- Steric hinderance lowers reactivity 1 > 3
- The bigger the group the more polarizable the more reactive
- Needs a negatively charge atom to be more reactive
- Oxygen is more electrophilic so is less nucleophilic than nitrogen becomes less reactive when comparing the two; Nitrogen becomes unstable more reactive