Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Review for Orgo 2 Exam (that i keep forgetting)
front 1 hydrates | back 1 gem diol reaction Reversible to an aldehyde/ketone Relatively stable by the electron-withdrawing groups |
front 2 Hemiacetal | back 2 OH and OR Can occur under acidic, basic, neutral conditions Reversible like hydrates |
front 3 Intramolecular Hemiacetal formation | back 3 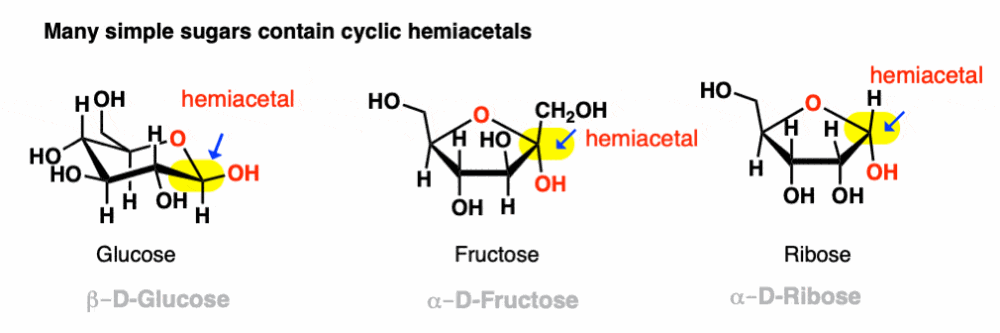 Alcohol + Aldehyde will most likely form a cyclic hemiacetal Cyclic hemiacetal can revert to acyclic alcohol by NaBH4 PADE P(protonation) A(addition) D(deprotonation) E(elimination) |
front 4 Reaction of Acetal | back 4 Are locked and not reversible so no reaction can take place after an acetal formation Undergo one reaction which is hydrolysis (H3O+ |
front 5 Acetal Protection Group | back 5 A Grignard reagent cannot be used with aldehydes or ketones, as it will react with them immediately. We can use ethylene glycol or Ch3OH H+ to protect |
front 6 Thioacetals | back 6 Raney Ni (reduces to alkanes) as well as the wolff-kisher and clemmensen |
front 7 Degree of Unsaturation | back 7 Formula: 2C+2+N-H-X/2 |
front 8 Hydrocarbon(no rings/double bonds) formula | back 8 Key Pattern formula: # Hydrogens = 2 x (# of Carbons) + 2 Pi bond reduce the hydrogen count by 2 |
front 9 IR spectroscopy | back 9 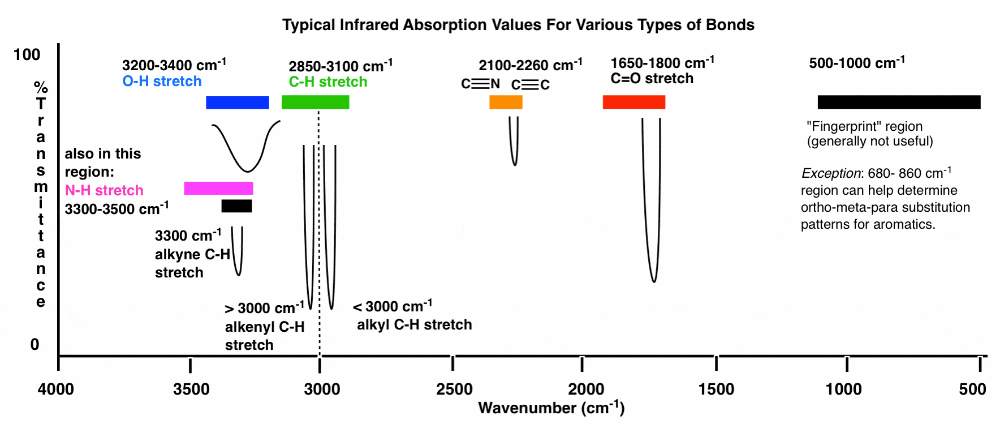 3600 – 2700 cm-1X-H (single bonds to hydrogen) 2700 – 1900 cm-1 X≡X (triple bonds) 1900 – 1500 cm-1 X=X (double bonds) 1500 – 500 cm -1X–X (single bonds) 3000 border between alkene(above) and alkane(below) dont look for degrees of unsat if there isnt any |
front 10 More important notes of IR | back 10 3400-3200 (OH appear) 1850 -1630 (C=O) [like swords] 3200 Amines and Amides Appear - Primary Amine: 2 stretched-out small peaks (like balls) - Secondary Amine: 1 long stretch peak - Primary Amide: 2 peaks a little more spaced out (like boobs - Secondary Amide: 1 long a little open peak further down (rare) Triple bond region around 2050-2250 cm-1 |
front 11 More info | back 11 Aldehydes(1740-1690) Ketones (1750-1680) Esters (1750 -1735) Carboxylic acids (1780 -1710) Amide (1690-1630) Anhydrides (1830-1800) (1775-1740) |
front 12 Monoasscharides | back 12 Glucose, Fructose |
front 13 Disaccharide | back 13 Sucrose, Maltose, Lactose |
front 14 Epimers | back 14 When two diastereomers only different from 1 carbon |
front 15 Fisher Projects for Glycosides | back 15 OH on the right (goes down) OH on the left (goes up) 5 carbon OH on the right is (D) 5 carbon OH on the left is (L) 5 and 6 carbon bonds rotates during glycoside formation and if OH is down, it does not become up. If up down |
front 16 Glycosides formation | back 16 Carbon 1: Up = Beta (more predominant) (more likely seen) Carbon 1: Down = Alpha |
front 17 Reducing Sugar for Carbohydrates | back 17 Hemiacetal and Aldehydes/ketones |
front 18 p - Toluidine | back 18 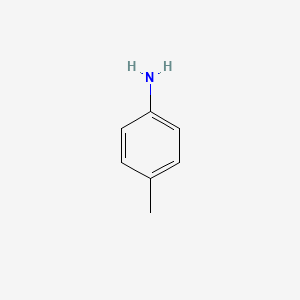 |
front 19 Morpholine | back 19 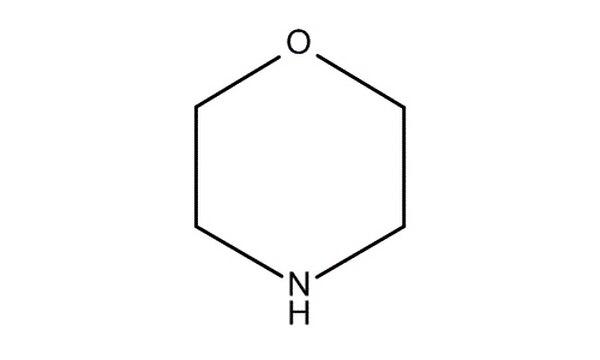 |
front 20 Pyrrolidine | back 20 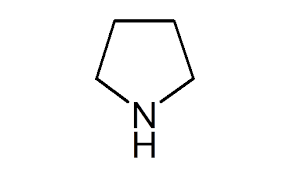 |
front 21 Diethylamine | back 21 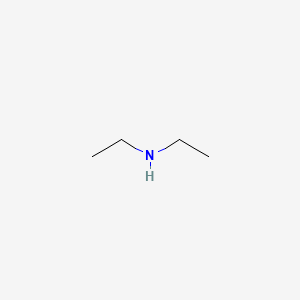 |
front 22 Acetophenone | back 22 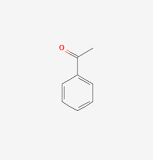 |
front 23 Lactone | back 23 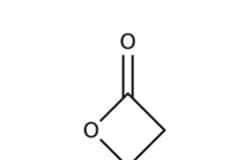 |
front 24 Ranking Nucleophiles | back 24 - Steric hinderance lowers reactivity 1 > 3 - The bigger the group the more polarizable the more reactive - Needs a negatively charge atom to be more reactive - Oxygen is more electrophilic so is less nucleophilic than nitrogen becomes less reactive when comparing the two; Nitrogen becomes unstable more reactive |