Chapter 5 - Stereochemistry
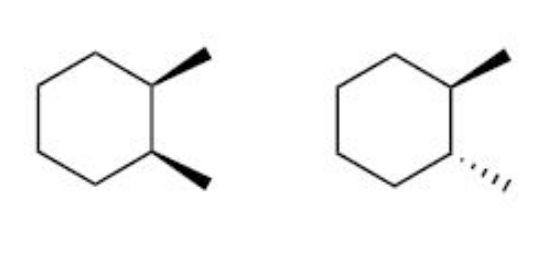
Determine the relationship between the following two compounds? Also name the compounds according to IUPAC rules.
Diastereomers
(cis)-1,2-dimethylcyclohexane
(trans)-1,2-dimethylcyclohexane
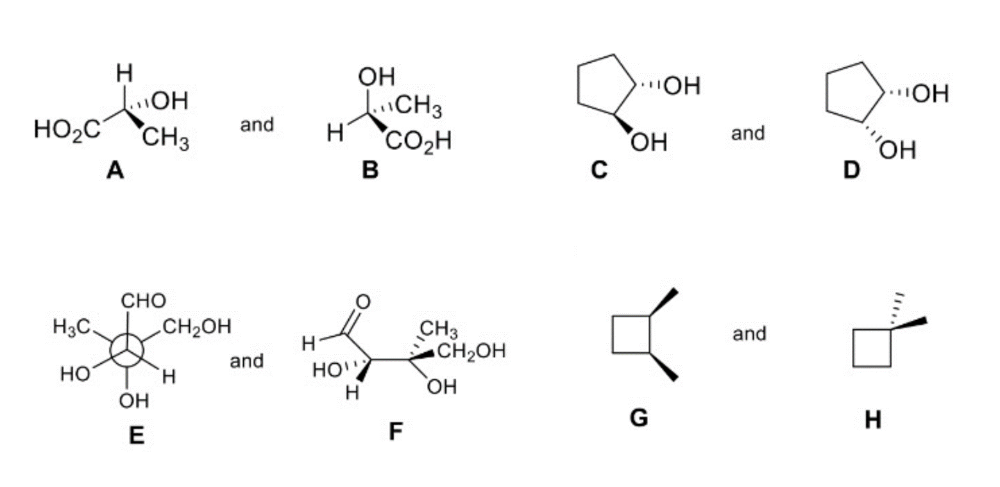
How are the compounds in each pair related to each other?
A and B are enantiomers
C and D are diasteromers
E and F are identical
G and H are constitutional isomers
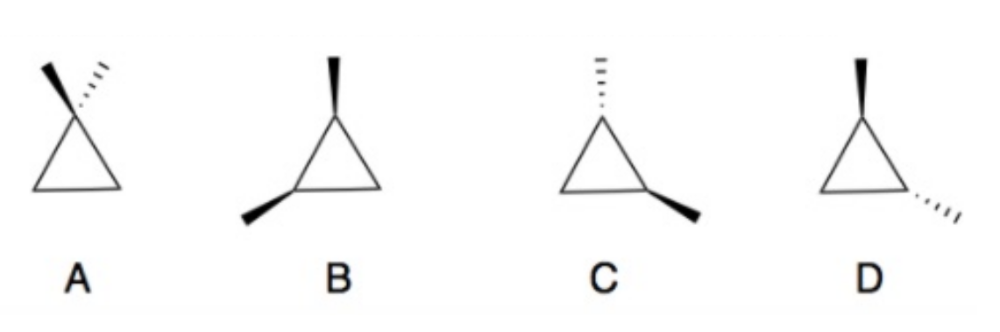
Determine which of the following compounds are achiral. If not, identify the stereogenic centers and label as R or S configuration.
A and B are achiral.
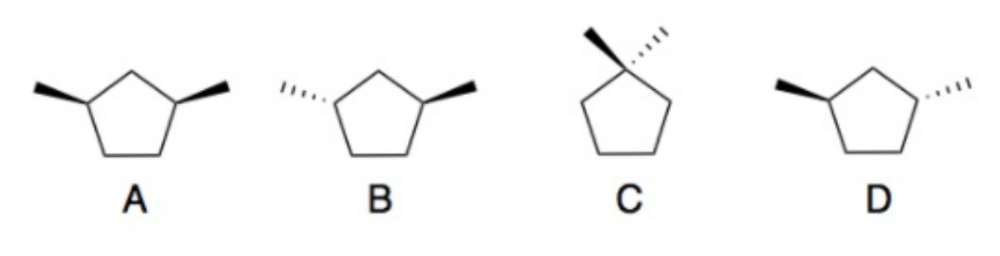
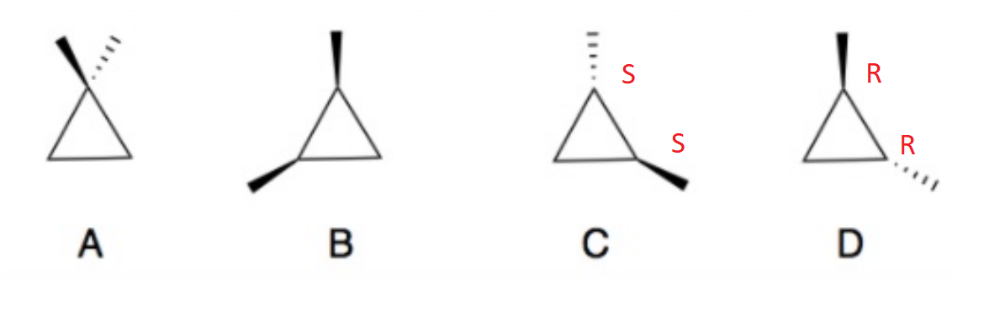
Determine which of the following compounds are optically active.
Compounds B and D are chiral, therefore optically active

Compare the following boiling points for compounds B and D
The boiling points for the two compounds are the same.
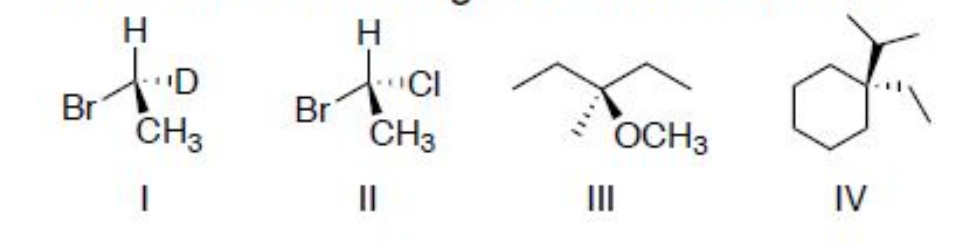
Determine which of the following molecules are chiral. If so, identify either R or S configuration.
I (R) and II (R) are chiral molecules.
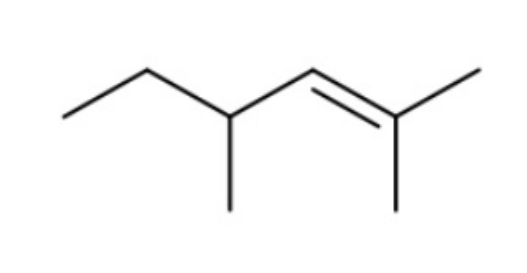
How many 1H and 13C NMR signals does the following compound have?
6 and 7 respectively
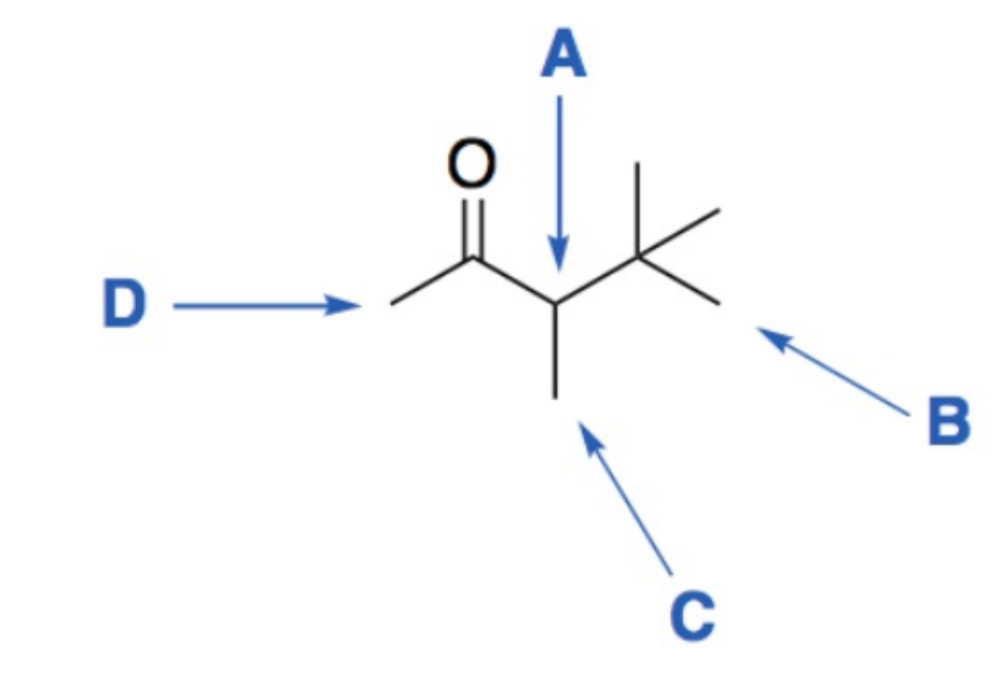
Determine the integration and splitting pattern for each proton signal.
A - (1H) quartet
B - (9H) singlet
C - (3H) doublet
D - (3H) singlet
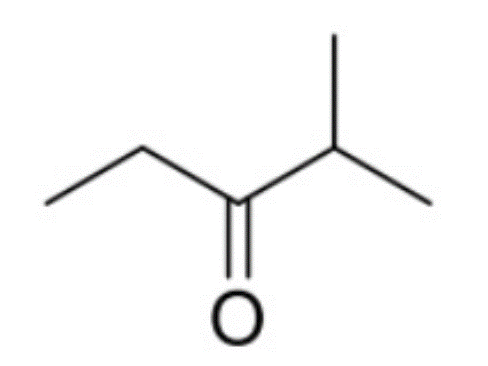
How many 1H and 13C NMR signals does the following compound have? Determine the integration and splitting pattern for each proton signal.
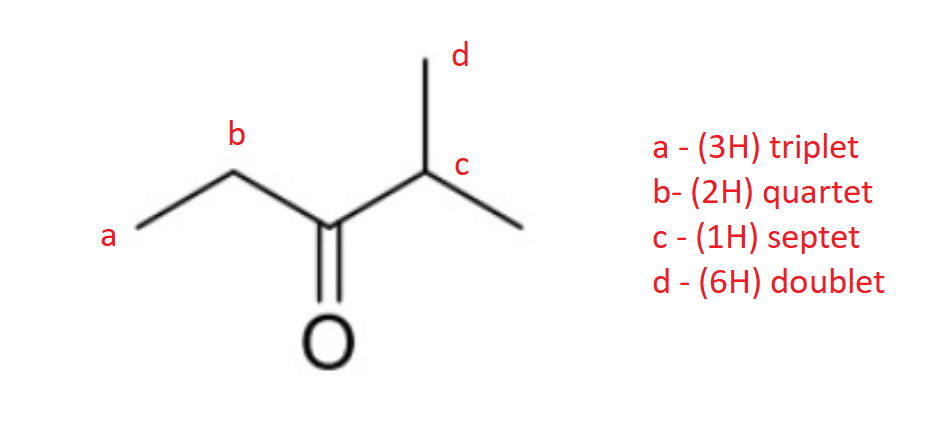
4 and 5 respectively.
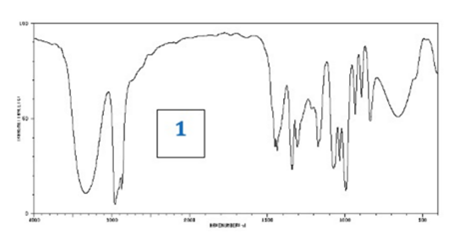
What information could be deduced from this IR Spectrum?
O-H group (strong broad peak around 3000)
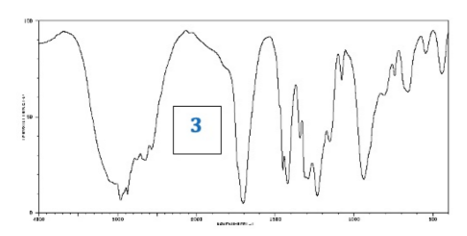
What information could be deduced from this IR Spectrum?
O-H Group (strong broad peak around 3000) and C=0 (strong peak at 1700)
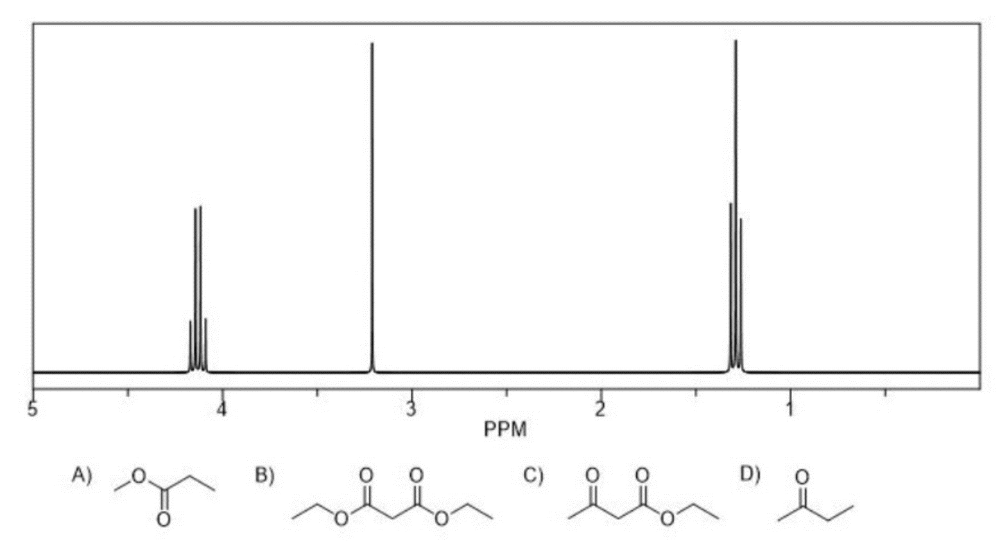
Which structure is most consistent with the following 1H NMR and which details allowed you to eliminate the other structures?
Structure B
A- The most downfield structure is a quartet, but the proton signal bonded directly to the oxygen is a singlet
C- There are three signals shown on the NMR whereas the structure has four signals
D- The signal on the NMR are more downfield than the protons shown on the compound