Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Chapter 5 - Stereochemistry
front 1 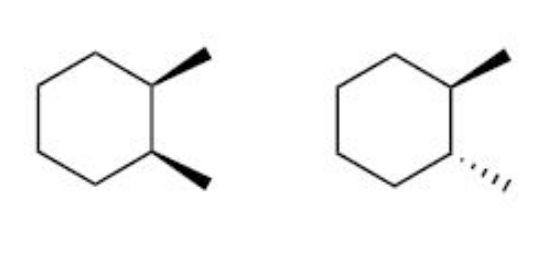 Determine the relationship between the following two compounds? Also name the compounds according to IUPAC rules. | back 1 Diastereomers (cis)-1,2-dimethylcyclohexane (trans)-1,2-dimethylcyclohexane |
front 2 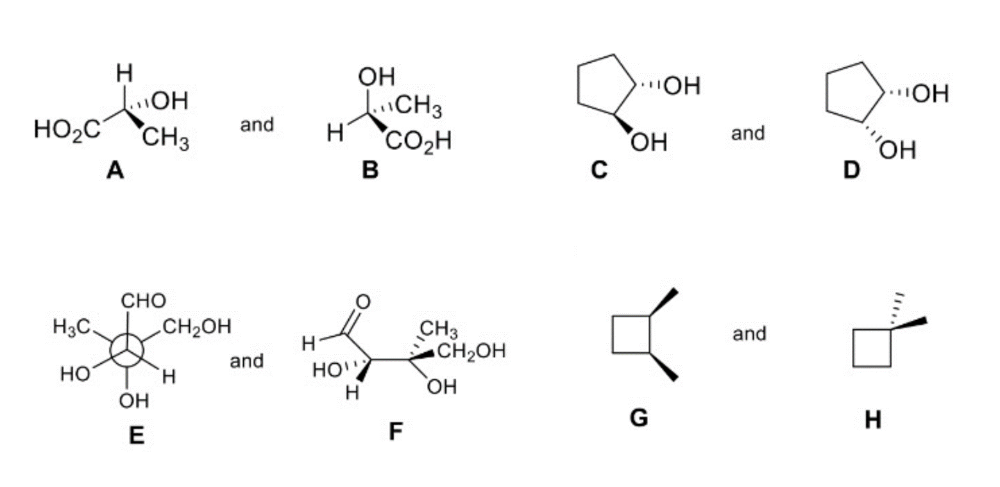 How are the compounds in each pair related to each other? | back 2 A and B are enantiomers C and D are diasteromers E and F are identical G and H are constitutional isomers |
front 3 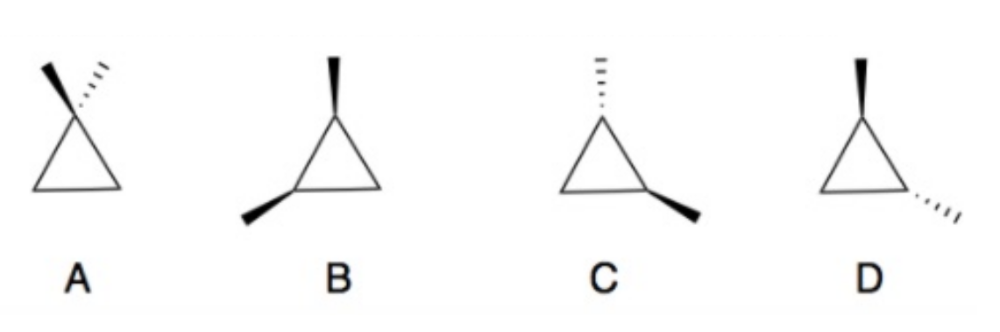 Determine which of the following compounds are achiral. If not, identify the stereogenic centers and label as R or S configuration. | back 3 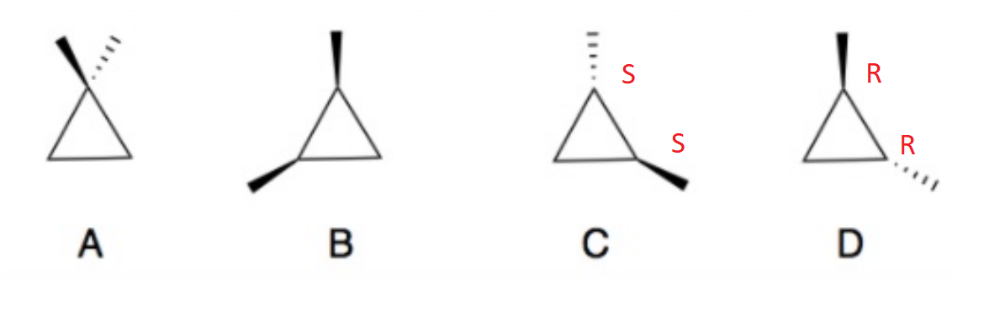 A and B are achiral. |
front 4 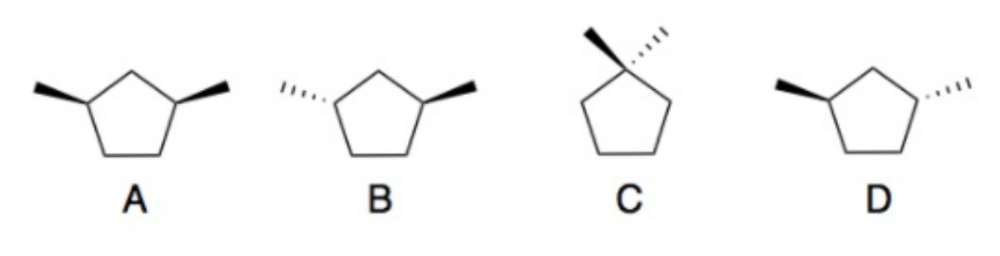 Determine which of the following compounds are optically active. | back 4 Compounds B and D are chiral, therefore optically active |
front 5  Compare the following boiling points for compounds B and D | back 5 The boiling points for the two compounds are the same. |
front 6 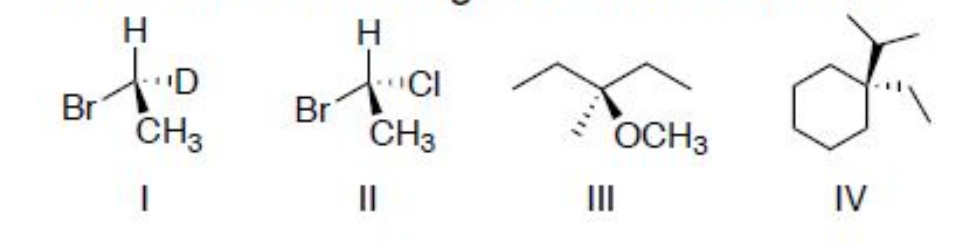 Determine which of the following molecules are chiral. If so, identify either R or S configuration. | back 6 I (R) and II (R) are chiral molecules. |
front 7 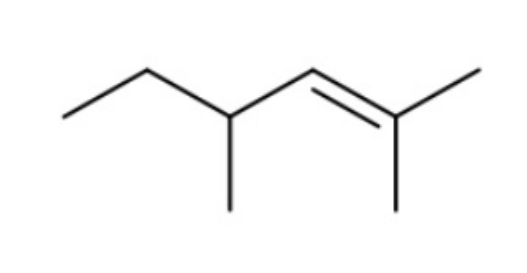 How many 1H and 13C NMR signals does the following compound have? | back 7 6 and 7 respectively |
front 8 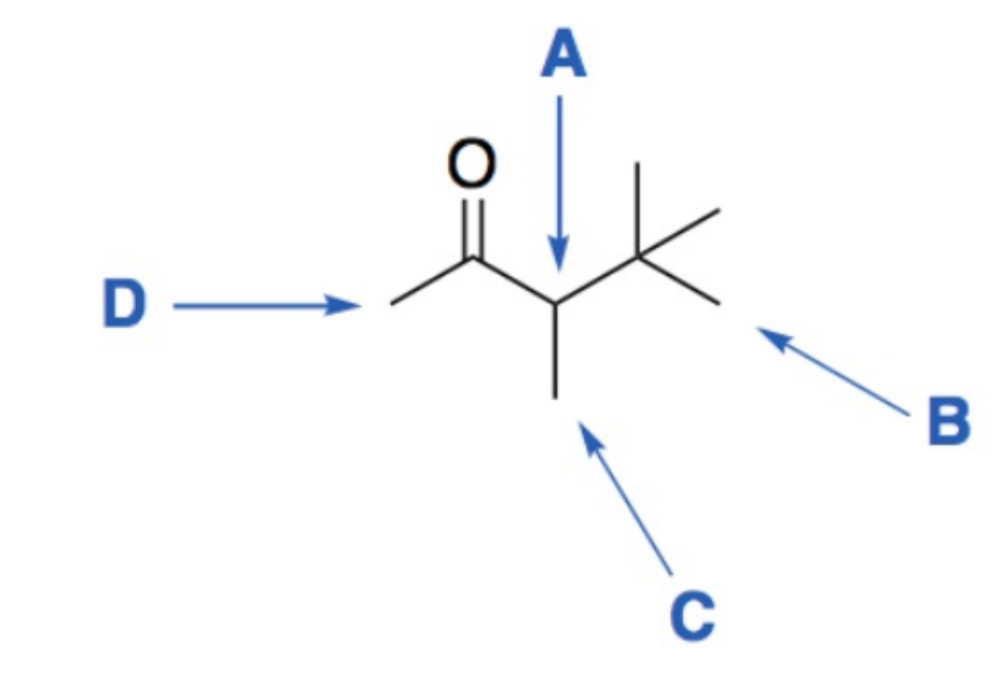 Determine the integration and splitting pattern for each proton signal. | back 8 A - (1H) quartet B - (9H) singlet C - (3H) doublet D - (3H) singlet |
front 9 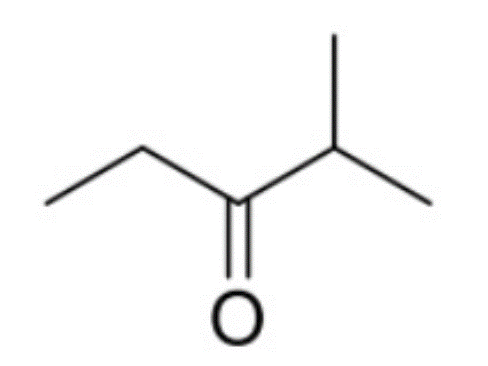 How many 1H and 13C NMR signals does the following compound have? Determine the integration and splitting pattern for each proton signal. | back 9 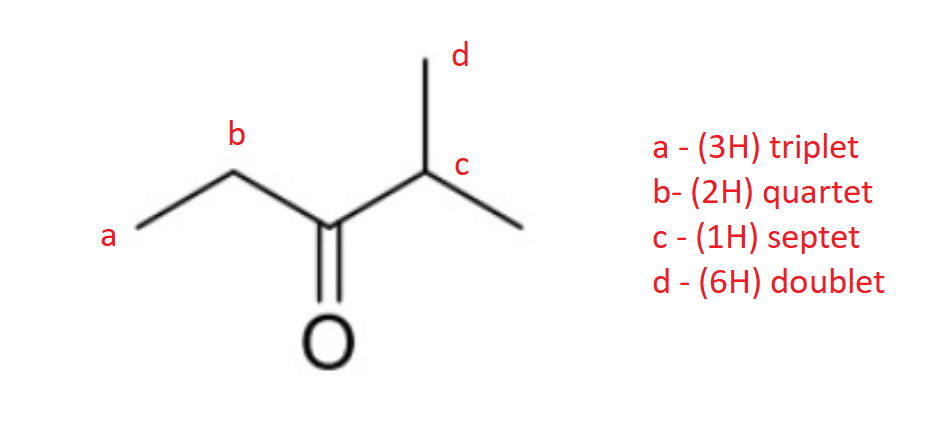 4 and 5 respectively. |
front 10 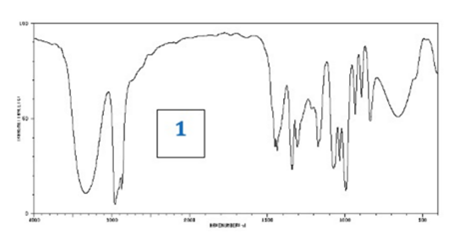 What information could be deduced from this IR Spectrum? | back 10 O-H group (strong broad peak around 3000) |
front 11 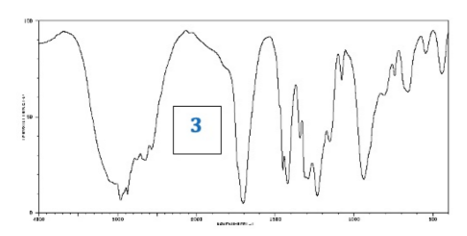 What information could be deduced from this IR Spectrum? | back 11 O-H Group (strong broad peak around 3000) and C=0 (strong peak at 1700) |
front 12 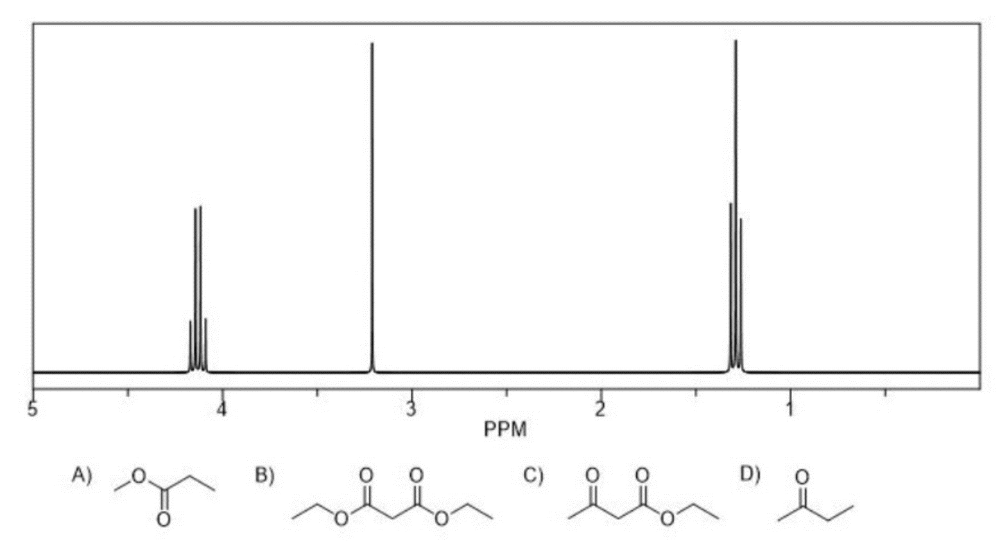 Which structure is most consistent with the following 1H NMR and which details allowed you to eliminate the other structures? | back 12 Structure B A- The most downfield structure is a quartet, but the proton signal bonded directly to the oxygen is a singlet C- There are three signals shown on the NMR whereas the structure has four signals D- The signal on the NMR are more downfield than the protons shown on the compound |