Chapter 4 - Alkanes
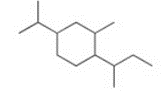
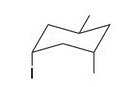
Give the IUPAC name for the following compound
1-sec-butyl-4-isopropyl-2-methylcyclohexane

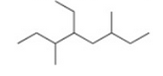
Give the IUPAC name for the following compound
3,5-diethyl-2-methylheptane
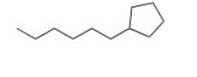
Give the IUPAC name for the following compound
1-cyclopentylhexane
Draw an appropriate structure given the IUPAC name of the following compound:
5-sec-butyl-3-ethyl-2,7-dimethyldecane

Draw an appropriate structure given the IUPAC name of the following compound:
4-ethyl-3,6-dimethyloctane
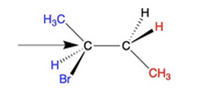
Convert the following structure to a Newman Projection. Rotate projection 60 degrees and identify which conformation is more stable.
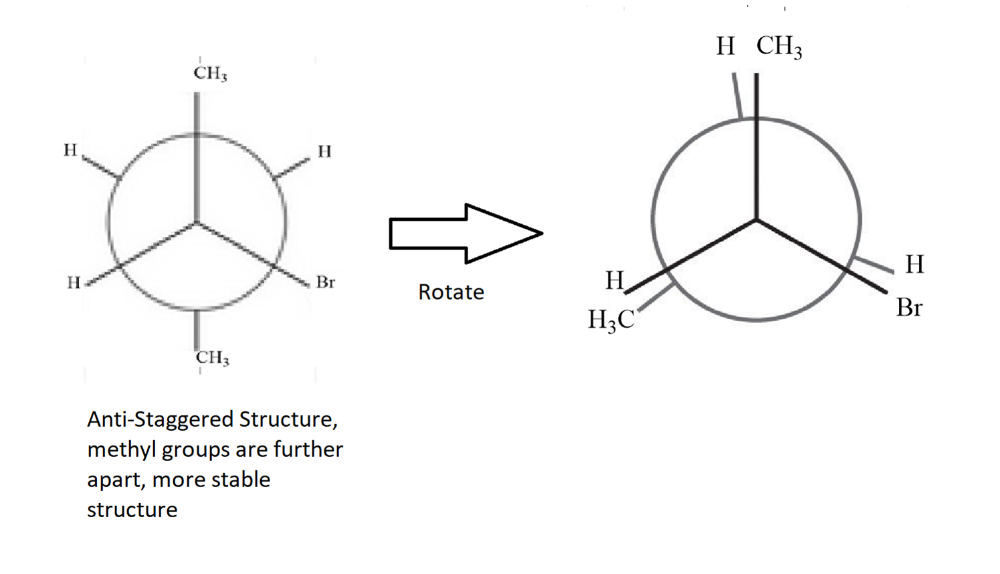
**The rotated conformation is neither the most or least stable. The least stable would have the methyl groups eclipsing each other.
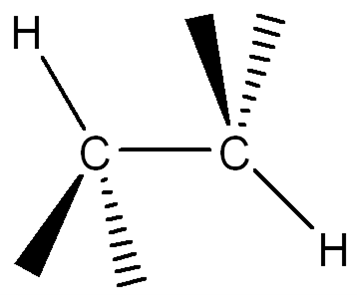
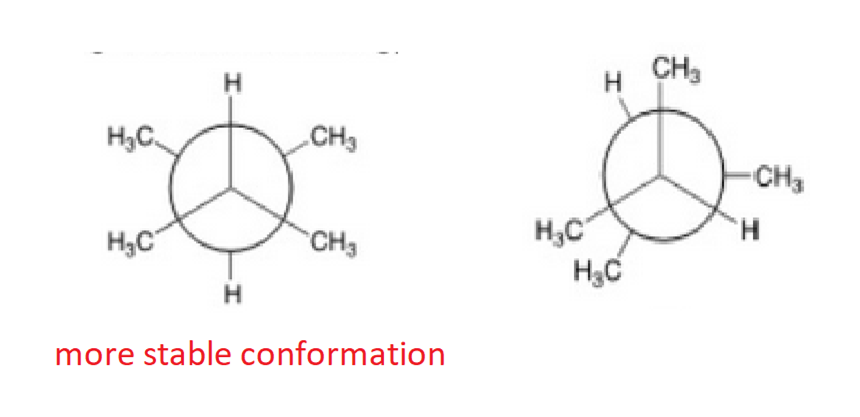
Convert the following structure to a Newman Projection (The eye is on the lefthand side). Rotate projection 60 degrees and identify which conformation is higher in energy.
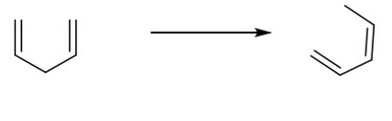
Classify the following reaction as oxidation, reduction, or neither.
Neither

Classify the following reaction as oxidation, reduction, or neither.
Oxidation
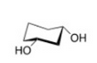
Draw another conformer of the structure provided of cis-cyclohexane-1,3-diol. Identify which is most stable.
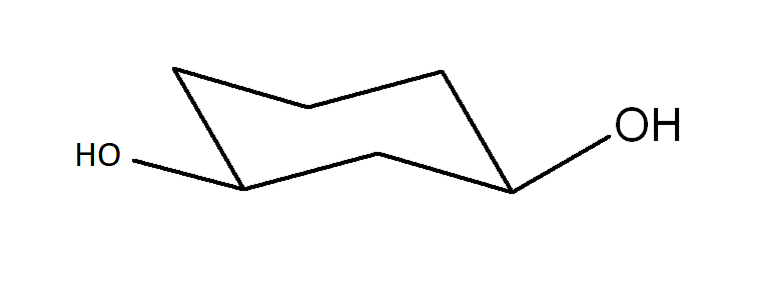
The conformer following the ring flip is more stable because the substituents (large groups) are avoiding 1,3-trans-diaxial interactions.
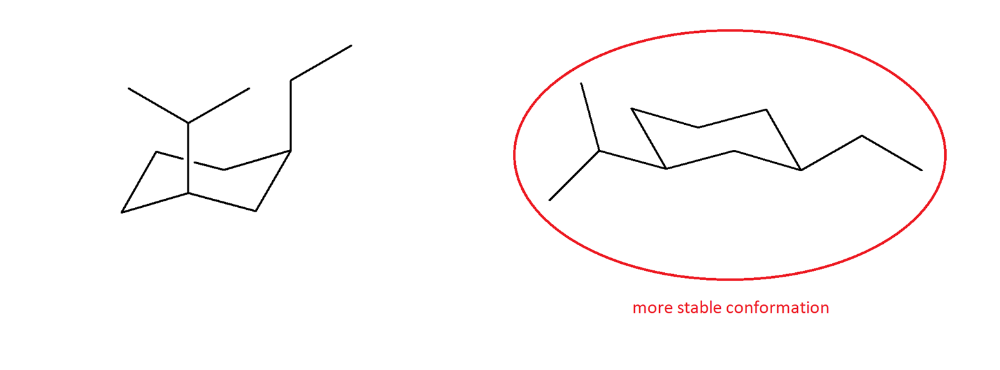
Draw an appropriate conformer for cis-1-ethyl-3-isopropylcyclohexane. Perform a ring flip, and then determine which is the least stable conformation.
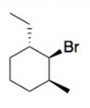
Draw a possible chair conformation for the substituted cyclohexane
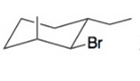

Draw a possible chair conformation for the substituted cyclohexane