Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Exam II
front 1 What includes all of the pyrimidines found in RNA and DNA? | back 1 cytosine, uracil, thymine |
front 2 Lactose, a sugar in milk, is composed of one glucose molecule joined by a glycosidic linkage to one galactose molecule. How is lactose classified? | back 2 as a disaccharide |
front 3 Which class of biological molecules does NOT include polymers? | back 3 lipids |
front 4 You disrupt all hydrogen bonds in a protein. What level of structure will be preserved? | back 4 primary structure |
front 5 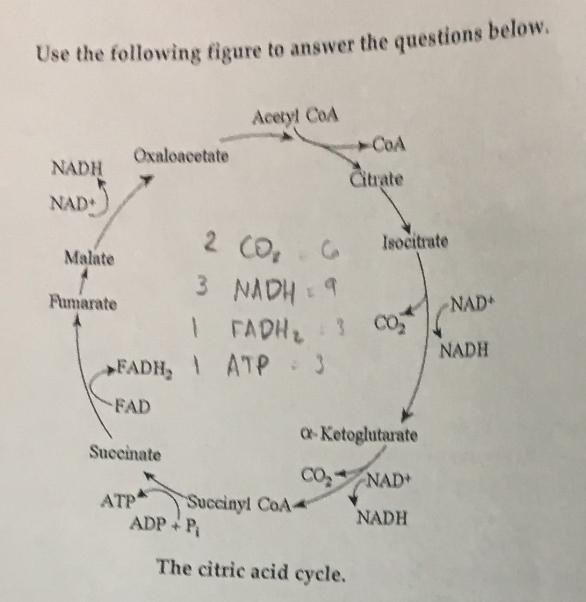 A fat (or triaclygycerol) would be formed as a result of a dehydration reaction between... | back 5 three molecules of 9 and one molecule of 10 |
front 6 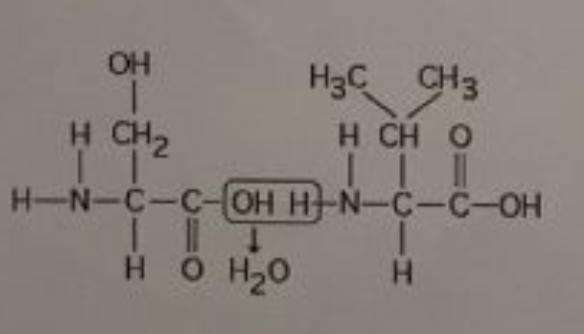 The chemical reaction illustrated in the accompanying figure___. | back 6 results in a peptide bond |
front 7 If one strand of a DNA molecule has the sequence of bases 5'ATTGCA3', the other complementary strand would have the sequence___. | back 7 5'TGCAAT3' |
front 8 What description best fits the class of molecules known as nucleosides? | back 8 a nitrogenous base and a sugar |
front 9 Which statement summarizes the relationship between dehydration reactions and hydrolysis? | back 9 Dehydration reactions assemble polymers; hydrolysis reactions break polymers apart |
front 10 Proteorhodopsin consists of a single polypeptide chain. What is the highest level of structure found in this protein? | back 10 tertiary |
front 11 The R-group, or side chain, of the amino acid serine is -CH2-OH. The R-group, or side chain, of the amino acid leucine is -CH2-CH-(CH3)2. Where would you expect to find these amino acids in a globular protein in aqueous solution? | back 11 Leucine would be in the interior, and serine would be on the exterior of the globular protein |
front 12 Why is glycogen extensively branched? | back 12 to be digested faster |
front 13 Which macromolecule leaves the nucleus of a eukaryotic cell thorugh pores in the nuclear membrane? | back 13 mRNA |
front 14 Which organelle is the primary state of ATP synthesis in eukaryotic cells? | back 14 Mitochondrion |
front 15 What is the most likely pathway taken by a newly synthesized protein that will be secreted by a cell? | back 15 ER --> Golgi --> vesicles that fuse with plasma membrane |
front 16 Which structure is common to plant and animal cells? | back 16 mitochondrion |
front 17 The advantage of light microscopy over electron microscopy is that___. | back 17 light microscopy allows one to view dynamic processes in living cells |
front 18 All of the following are part of a prokaryotic cell EXCEPT___. | back 18 an endoplasmic reticulum |
front 19 What produces and modifies polysaccharides that will be secreted? | back 19 Golgi apparatus |
front 20 A cell with a predominance of free ribosomes is most likely... | back 20 primarily producing proteins in the cytosol |
front 21 Where would you expect to find tight junctions? | back 21 in the plasma membrane of prokaryotes |
front 22 Ions can travel directly from the cytoplasm of one animal cell to the cytoplasm of an adjacent cell through... | back 22 gap junctions |
front 23 Which statement correctly describes a prokaryotic cell's length? | back 23 10,000 nano-meter. |
front 24 A sperm would be unable to swim if it does not have___. | back 24 Ribosome, actin, and centrosome. |
front 25 Why are lipids and proteins free to move laterally in membranes? | back 25 There are only weak hydrophobic interactions in the interior of the membrane |
front 26 The membranes of winter wheat are able to remain fluid when it is extremely cold by ___. | back 26 increasing the percentage of unsaturated phospholipids in the membrane |
front 27 What will happen to a red blood cell (RBC), which has an internal ion concentration of about 0.9 percent, if it is placed into a beaker of pure water? | back 27 the cell would swell because the water in the beaker is hyoptonic relative to the cytoplasm of the RBC. |
front 28 In some cells, there are many ion electrochemical gradients across the plasma membrane even though there are usually only one or two proton pumps present in the membrane. The gradients of the other ions are most likely accounted for by ___. | back 28 passive diffusion across the plasma membrane |
front 29 What is NOT amphipathic? | back 29 the surface of peripheral proteins |
front 30 What kinds of molecules pass through a cell membrane most easily? | back 30 small and hydrophobic |
front 31 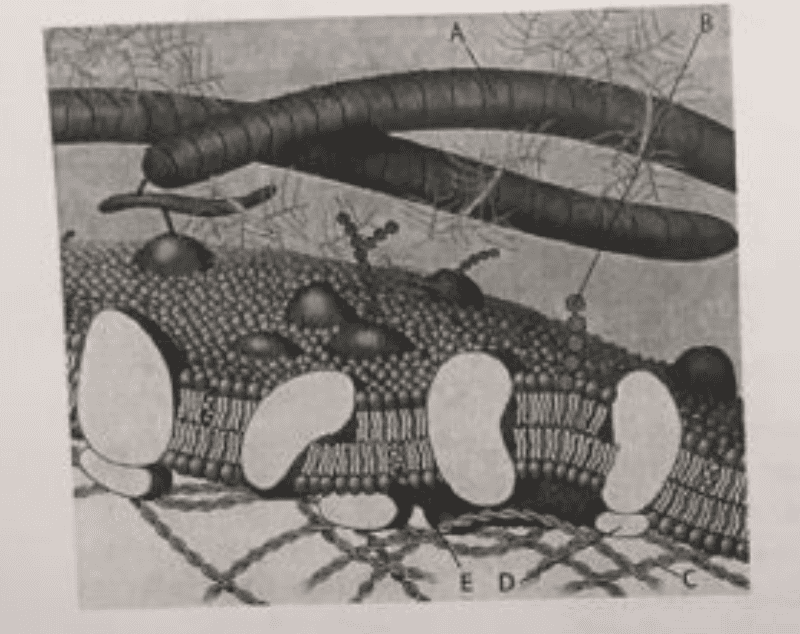 Which component is a peripheral protein? | back 31 D |
front 32 White blood cells engulf bacteria using___. | back 32 Phagocytosis |
front 33 Which term most precisely describes the cellular process of breaking down large molecules into smaller ones? | back 33 Catabolism (catabolic pathways) |
front 34 A chemical reaction that has a positive ΔG is best desribed as... | back 34 endergonic |
front 35 What is an example of potential rather than kinetic energy? | back 35 a molecule of glucose |
front 36 Which of the following is true of enzymes? | back 36 enzymes increase the rate of chemical reaction by lowering energy barriers. |
front 37 Which of the following involves a decrease in entropy? | back 37 condensation reactions |
front 38 A noncompetitive inhibitor decreases the rate of enzyme reaction by... | back 38 changing the shape of the enzyme's active site |
front 39 The mathematical expression for the change in free energy of a system is ΔG= ΔH-TΔS. Which of the following is correct? | back 39 ΔG is the change in free energy. |
front 40 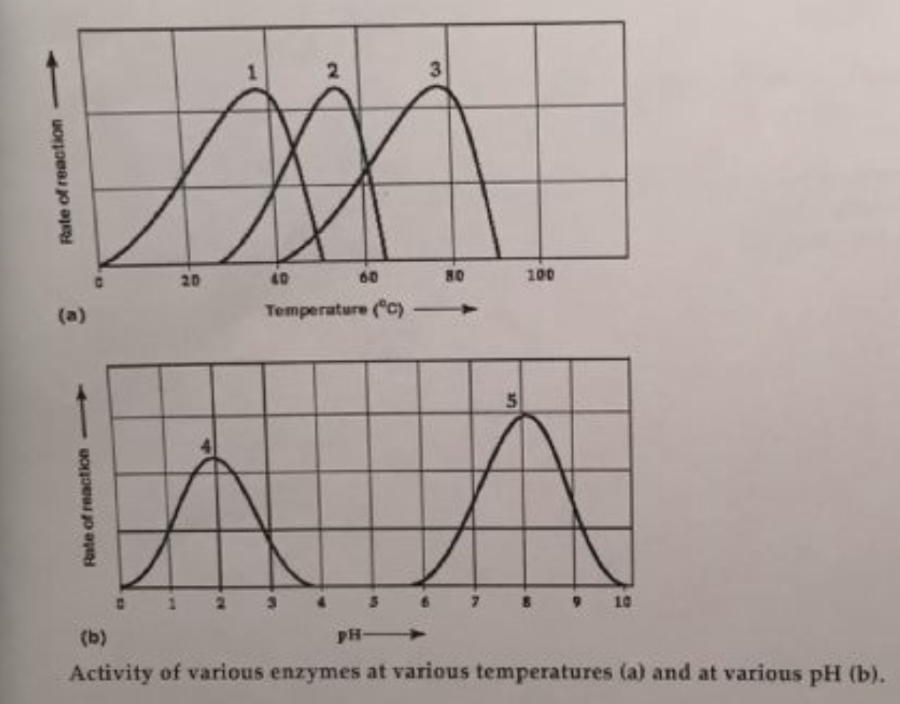 Which temperature and pH profile curves on the graphs were most likely generated from analysis of an enzyme from a human stomach where conditions are strongly acid? | back 40 curves 3 and 4 |
front 41 Which of the following statements is a logical consequence of the second law of thermodynamics? | back 41 every chemical reaction must increase the total entropy of the universe |