Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
LECTURE test 1 review bio 1406
front 1 A controlled experiment | back 1 Control group |
front 2 A localized group of organisms that belong to the same species is called a | back 2 A population |
front 3 You find yourself standing next to a plant. List the things you and the plant have in common? | back 3 use oxygen and carbon to survive, are linear, |
front 4 A friend of yours calls to say that his car would not start this morning. He asks for your help. You say that you think the battery must be dead. If so, then jump-starting the car from a good battery will solve the problem. In doing so, you are doing what part of the scientific process? | back 4 hypothesis |
front 5 In the process of science, what do you test? | back 5 collected data |
front 6 What four elements make up approximately 96 percent of living matter? | back 6 hydrogen, oxygen, carbon, and nitrogen |
front 7 Trace elements are those required by an organism in only minute quantities. Which trace element that is required by humans and other vertebrates for normal thyroid function. | back 7 Iodine |
front 8 A covalent chemical bond is one in which | back 8 outer-shell electrons of two atoms are shared so as to satisfactorily fill the outer electron shells of both atoms. |
front 9 What results from an unequal sharing of electrons between atoms? | back 9 a polar covalent bond |
front 10 Â Explain the difference between covalent bonds and ionic bonds? | back 10 one of the atoms sharing electrons is more electronegative than the other atom. |
front 11 Water molecules are attracted to one another by | back 11 Hydrogen Bond |
front 12 How many electrons are involved in a single covalent bond? | back 12 a single covalent bond has 2 atoms which hold 1 electron each atom 2 |
front 13 An carbon atom has four electrons in its valence shell. What types of covalent bonds is it capable of forming? | back 13 single, double, or triple |
front 14 When the atoms involved in a covalent bond have the same electronegativity, what type of bond results? | back 14 a nonpolar covalent bond |
front 15 In a single molecule of water, two hydrogen atoms are bonded to a single oxygen atom by | back 15 a polar covalent bond |
front 16 The partial negative charge at one end of a water molecule is attracted to the partial positive charge of another water molecule. What is this attraction called? | back 16 a hydrogen bond |
front 17 Explain why does water display a partial negative charge? | back 17 electrons shared between the oxygen and hydrogen atoms spend more time around the oxygen atom nucleus than around the hydrogen atom nucleus. |
front 18 To act as an effective coolant in a car's radiator, a substance has to have the capacity to absorb a great deal of heat. You have a reference book with tables listing the physical properties of many liquids. In choosing a coolant for your car, which table would you check first? | back 18 specific heat |
front 19 Which of the following effects can occur because of the high surface tension of water? | back 19 A water strider can walk across the surface of a small pond. |
front 20 Hydrophobic substances such as vegetable oil are | back 20 nonpolar substances that repel water molecules |
front 21 Which type of bond must be broken for water to vaporize into a gas? | back 21 hydrogen bonds |
front 22  Label the below diagram: water, oxygen, hydrogen, partial negative charge, partial positive charge, solute | back 22 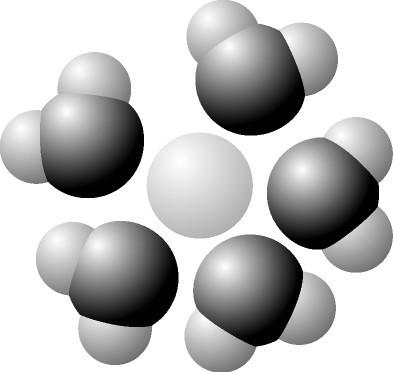 hydrogen oxygen na- the rest is positively charged |
front 23 How would you bring an acidic solution to neutral? | back 23 you have a weak acid or a weak base half ionized in water |
front 24 Why are molecules considered organic? | back 24 Organic molecules exist in all living things |
front 25 What types of covalent bonds can carbon form? | back 25 carbon-carbon and carbon hydrogen bonds |
front 26 Why is carbon so important in biology? | back 26 It can form a variety of carbon skeletons and host functional groups |
front 27 The complexity and variety of organic molecules is due to | back 27 the chemical versatility of carbon atoms |
front 28 A carbon atom is most likely to form what kind of bond(s) with other atoms? | back 28 covalent |
front 29 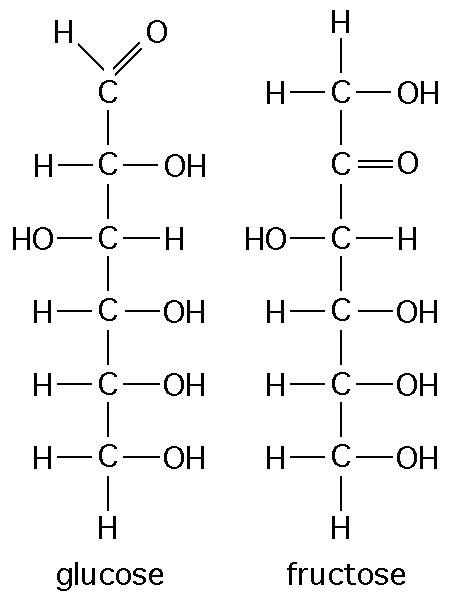 The figure above shows the structures of glucose and fructose. They both have the molecular formula C6H12O6, but how do they differ. | back 29 structural isomers |
front 30 Which two functional groups are always found in amino acids? Draw these functional groups. | back 30 carboxyl and amino |
front 31 Which class of organic molecules does NOT include polymers? | back 31 Lipids |
front 32 List all polymers discussed in class and the group in which they belong. | back 32 1.carbohydrates, |
front 33 Contrast dehydration reactions and hydrolysis? | back 33 Dehydration means to take water out. Hydrolysis is the separation of two macromolecules by adding water. |
front 34 Which polysaccharide is an important component in the structure of many animals and fungi? | back 34 Chitin |
front 35 A molecule with the chemical formula C6H12O6Â would be considered a what? | back 35 glucose carbohydrate and monosaccharide only |
front 36 List all things lactose can be considered. | back 36 as a disaccharide |
front 37 Â In what types of organisms are starch and cellulose found? What is their function? | back 37 They are both polymers of glucose |
front 38 List the types of covalent bonds discussed in class and the organic group in which they belong. | back 38 no data |
front 39 Phospholipids and triglycerides both | back 39 lipids |
front 40 Describe how the structure of phospholipids interacts with water molecules. | back 40 no data |
front 41 Explain why chemically vegetable oil is a liquid at room temperature while animal fats are solid. | back 41 no data |
front 42 Contrast saturated and unsaturated fatty acids. | back 42 no data |
front 43 List terms that describe how lipids behave in water. | back 43 no data |
front 44 What are the monomers of proteins? | back 44 Nucleotides |
front 45 List the components of an amino acid. | back 45 Components: amino, carboxyl, H, R (variable) group |
front 46 What component of amino acid structure varies among different amino acids? | back 46 no data |
front 47 Describe how protein structure and function are correlated? How can the structure of a protein be altered? | back 47 no data |
front 48 List the examples of proteins and lipids discussed in class. | back 48 no data |
front 49 Discuss the levels of protein structure folding. | back 49 primary structurelinear aequence of amino acids secondary structurealpha helix and beta pleated sheet formed by hudrogen bonds between atoms of the polypeptide backbone tertiary structure3d shape formed by interactions between r groups quaternary structureassociation of multiple polypeptides |
front 50 What are the monomers of nucleic acids? | back 50 nucleotides ribonucleotide Deoxyribonucleotide |
front 51 List the purine nucleotides. | back 51 nitrogenous bases found in DNA and RNA; either adenine or guanine |
front 52 List the pyrimidine nucleotides. | back 52 nitrogenous bases found in DNA and RNA; thymine, cytosine, or uracil |
front 53 List the components of a nucleotide? | back 53 A sugar (called deoxyribose) A Phosphate (1 phosphorus atom joined to 4 oxygen atoms) One of 4 bases (Adenine, Guanine, Cytosine, Thymine) |
front 54 What is the primary functions of RNA? | back 54 1. Messanger RNA (mRNA) |
front 55 When nucleotides polymerize to form a nucleic acid what bonds are formed | back 55 a covalent bond forms between the sugar of one nucleotide and the phosphate of a second |
front 56 Contrast DNA and RNA. | back 56 While the sugar present in a RNA molecule is ribose, the sugar present in a molecule of DNA is deoxyribose |
front 57 If one strand of a DNA molecule has the sequence of bases, ATTGCA, the other complementary strand would have the sequence | back 57 TAACGT |
front 58 A major type of lipid found in cell membranes is | back 58 Phospholipids |
front 59 You now know that the old cliché "oil and water don't mix" is true. Why? | back 59 Water exhibits polarity and oil does not. |
front 60 If you eat a hamburger, you are mainly eating ground-up beef muscle tissue. What levels of organization are represented in this ground-up muscle? | back 60 Organelle, cell, and tissue |
front 61 If you change the number of neutrons in an atom, you create | back 61 an isotope |
front 62 Bonds between two atoms that are equally electronegative are | back 62 nonpolar covalent bonds |
front 63 A covalent bond is likely to be polar when | back 63 one of the atoms sharing electrons is much more electronegative than the other atom. |
front 64 Water molecules can form hydrogen bonds with | back 64 compounds that have polar covalent bonds. |
front 65 What can be attributed to water's high specific heat? | back 65 A lake heats up more slowly than the air around it. |
front 66 Which type of bond must be broken for water to vaporize? | back 66 hydrogen bonds |
front 67 A strong acid like HCl dissociates | back 67 ionizes completely in an aqueous solution. |
front 68 How many electron pairs does carbon share to complete its valence shell? | back 68 4 |
front 69 How many molecules of water are used to completely hydrolyze a polymer that is 11 monomers long? | back 69 10 |
front 70 Steroids are considered to be lipids because they | back 70 A family of lipids distinguished by a bulky four-ring structure |
front 71 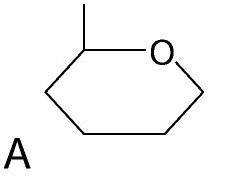 | back 71 Monosaccharide Carbohydrate |
front 72  | back 72 Protein |
front 73 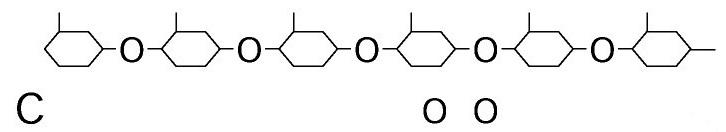 | back 73 Polysaccharides |
front 74 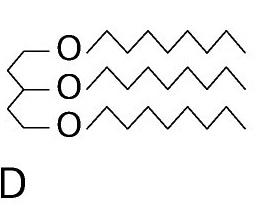 | back 74 triglyceride |
front 75 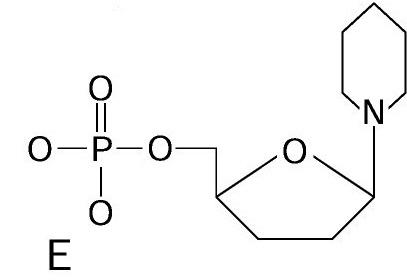 | back 75 Nucleotide |
front 76  | back 76 Lipid and Fatty acid |
front 77 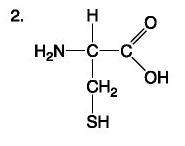 | back 77 Amino Acid |
front 78 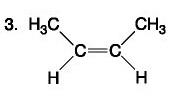 | back 78 Carbohydrate |
front 79 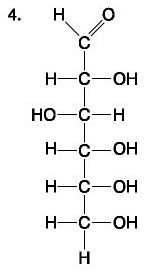 | back 79 Lipid and Fatty acid |
front 80 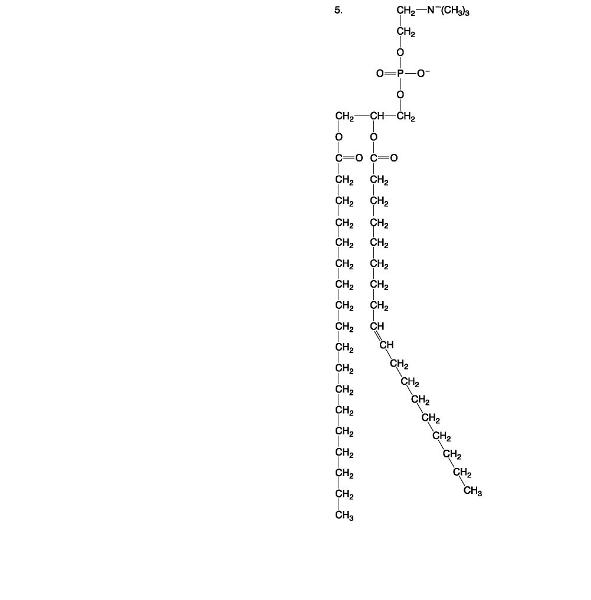 | back 80 Phospholipid |
front 81 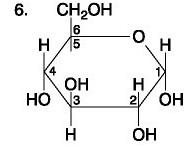 | back 81 Carbohydrate |
front 82 no data | back 82 Amino acid |
front 83 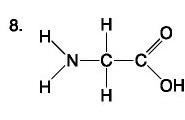 | back 83 Amino Acid |
front 84 no data | back 84 lipid and fatty acid |
front 85  | back 85 no data |
front 86 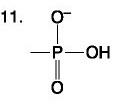 | back 86 Nucleotide |
front 87 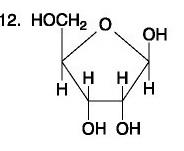 | back 87 no data |
front 88 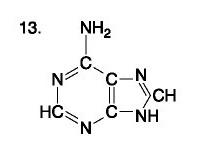 | back 88 no data |
front 89 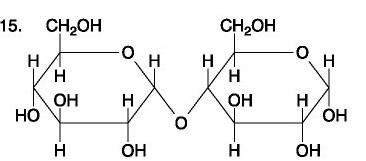 | back 89 no data |