Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
quiz 2
front 1 Choose the molecule or compound that exhibits dipole-dipole forces as its strongest intermolecular force. SO2 H2 CF4 BCl3 NH3 | back 1 SO2 |
front 2 Choose the pair of substances that are most likely to form a homogeneous solution. LiF and C6H14 C3H8 and C2H5OH Br2 and PF3 NH3 and CH3OH NaCl and Hg | back 2 NH3 and CH3OH |
front 3 Choose the substance with the highest boiling point. CS2 HF I2 KI CH4 | back 3 KI |
front 4 Choose the substance with the lowest surface tension. CH3CH2CH2CH3 H2O C6H6 (CH3)2CO CH3SH | back 4 CH3CH2CH2CH3 |
front 5 Choose the substance with the lowest vapor pressure at a given temperature. He BF3 BeCl2 CO2 PF5 | back 5 BeCl2 |
front 6 Choose the substance with the lowest viscosity. Cl2CHCH2Cl Cl3CCCl3 Cl3CCHCl2 ClCH2CH2Cl Cl2CHCHCl2 | back 6 ClCH2CH2Cl |
front 7 Give the change in condition to go from a liquid to a gas. Cool or reduce pressure Increase heat or reduce pressure Cool or increase pressure Increase heat or increase pressure None of the above | back 7 Increase heat or reduce pressure |
front 8 Give the characteristic of a first order reaction having only one reactant. The rate of the reaction is directly proportional to the concentration of the reactant. The rate of the reaction is proportional to the natural logarithm of the concentration of the reactant. The rate of the reaction is proportional to the square root of the concentration of the reactant. The rate of the reaction is not proportional to the concentration of the reactant. The rate of the reaction is proportional to the square of the concentration of the reactant. | back 8 The rate of the reaction is directly proportional to the concentration of the reactant. |
front 9 Give the term for the temperature at which the gas and liquid phases form a supercritical fluid. Definite temperature. Absolute temperature. Critical temperature. Fluid temperature. Solid temperature | back 9 Critical temperature. |
front 10 Given the following balanced equation, determine the rate of reaction
with respect to [NOCl]. If the rate of Cl2 loss is 4.84 ×
10-2 M/s, what is the rate of formation of NOCl? Answers: 4.84 × 10-2 M/s 1.61 × 10-2 M/s 1.45 × 10-1 M/s 9.68 × 10-2 M/s 2.42 × 10-2 M/s | back 10 9.68 × 10-2 M/s |
front 11 Given the following rate law, how does the rate of reaction change if
the concentration of X is halved and the concentration of Y is
doubled? The rate of reaction will increase by a factor of 5. The rate of reaction will increase by a factor of 2. The rate of reaction will decrease by a factor of 2. The rate of reaction will increase by a factor of 4. The rate of reaction will remain unchanged. | back 11 The rate of reaction will increase by a factor of 2. |
front 12 How many half-lives are required for the concentration of reactant to decrease to 12.5% of its original value? 2 1.75 3 2.75 1 | back 12 3 |
front 13 How much energy is required to vaporize 48.7 g of dichloromethane (CH2Cl2) at its boiling point, if its ΔHvap is 31.6 kJ/mol? 18.1 kJ 55.1 kJ 31.2 kJ 15.4 kJ 6.49 kJ | back 13 18.1 kJ |
front 14 Place the following compounds in order of
decreasing
strength of intermolecular forces. HF > O2 > CO2 O2 > CO2 > HF CO2 > HF > O2 HF > CO2 > O2 CO2 > O2 > HF | back 14 HF > CO2 > O2 |
front 15 Place the following compounds in order of
increasing
strength of intermolecular forces. NH2CH3 < F2 < CO2 NH2CH3 < CO2 < F2 F2 < CO2 < NH2CH3 CO2 < NH2CH3 < F2 F2 < NH2CH3 < CO2 | back 15 F2 < CO2 < NH2CH3 |
front 16 What are the units of k in a first order reaction? | back 16 1/s |
front 17 What are the units of k in the following rate law? | back 17 M-1s-1 |
front 18 What data should be plotted to show that experimental concentration data fits a second-order reaction? 1/[reactant] vs. time ln[reactant] vs. time ln(k) vs. Ea ln(k) vs. 1/T [reactant] vs. time | back 18 1/[reactant] vs. time |
front 19 What is the overall order of the following reaction, given the rate
law? 1st order 0th order 2nd order 5th order 3rd order | back 19 3rd order |
front 20 What is the overall order of the following reaction, given the rate
law? 0th order 3rd order 4th order 2nd order 1st order | back 20 3rd order |
front 21 What is the strongest type of intermolecular force present in CHF3? hydrogen bonding ion-dipole dipole-dipole dispersion None of the above | back 21 dipole-dipole |
front 22 What is the strongest type of intermolecular force present in H2? dispersion hydrogen bonding ion-dipole dipole-dipole None of the above | back 22 dispersion |
front 23 What is the strongest type of intermolecular force present in NH2CH3? dipole-dipole ion-dipole dispersion hydrogen bonding None of the above | back 23 hydrogen bonding |
front 24 represents the equation for a first-order half-life? | back 24 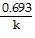 t 1/2 = |
front 25 represents the integrated rate law for a first-order reaction? | back 25 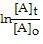 = -kt |
front 26 Which of the following statements is TRUE? Until a certain point, the potential energy of molecules decrease as they get closer to one another. Intermolecular forces are generally stronger than bonding forces. Increasing the pressure on a solid usually causes it to become a liquid. Energy is given off when the attraction between two molecules is broken. None of the above are true. | back 26 Until a certain point, the potential energy of molecules decrease as they get closer to one another. |
front 27 Which substance below has the strongest intermolecular forces? C3X2, ΔHvap= 36.4 kJ/mol DX2, ΔHvap= 23.3 kJ/mol BY2, ΔHvap= 26.7 kJ/mol A2X, ΔHvap= 39.6 kJ/mol EY3, ΔHvap= 21.5 kJ/mol | back 27 A2X, ΔHvap= 39.6 kJ/mol |