Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Final Exam
front 1 When applying the process of science, which of these is tested? | back 1 Hypothesis |
front 2 About 25 of the 92 natural elements are known to be essential to life. Which 4 of these 25 elements make up approximately 96% of living matter? | back 2 carbon, hydrogen, nitrogen, oxygen |
front 3 Which of the following is not a common life property? | back 3 Inductive Reasoning |
front 4 Why does ice float in liquid water? | back 4 Hydrogen bonds stabilize and keep the molecules of ice farther apart than the water molecules of liquid water. The crystalline lattice of ice causes it to be denser than liquid water. |
front 5 A water sample from a hot thermal vent contained a single-celled organism that had a cell wall but lacked a nucleus. What is its most likely classification? | back 5 Archaea |
front 6 The first electron shell can be filled with a maximum of _________ electrons. All subsequent shells can be filled with a maximum of _________ electrons. | back 6 2,8 |
front 7 One liter of a solution of pH 2 has how many more hydrogen ions (H+) than 1 L of a solution of pH 6? | back 7 10,000 times more |
front 8 What results from an unequal sharing of electrons between atoms? | back 8 A polar covalent bond |
front 9 Knowing just the atomic mass of an element allows inferences about which of the following? | back 9 the number of protons plus neutrons in the element |
front 10 Which of the following statements is true about buffer solutions? | back 10 They maintain a relatively constant pH when either acids or bases are added to them. |
front 11 Which of the following is not a polymer? | back 11 Starch |
front 12 Which of the following best summarizes the relationship between dehydration reactions and hydrolysis? | back 12 Dehydration reactions assemble polymers, and hydrolysis reactions break down polymers. |
front 13 Which of the following is true of both starch and cellulose? | back 13 They are both polymers of glucose. |
front 14 Testosterone and estradiol are male and female sex hormones, respectively, in many vertebrates. In what way(s) do these molecules differ from each other? | back 14 Testosterone and estradiol have different functional groups attached to the same carbon skeleton. |
front 15 Which bonds are created during the formation of the primary structure of a protein? | back 15 Peptide bonds |
front 16 Which level of protein structure do the α helix and the β pleated sheet represent? | back 16 secondary |
front 17 Which of the following descriptions best fits the class of molecules known as nucleotides? | back 17 a nitrogenous base, a phosphate group, and a pentose sugar |
front 18 Denaturation of proteins can occur if there is a change in ____________. | back 18 pH |
front 19 The Central Dogma: | back 19 0.0015 |
front 20 How does a raft spider manage to walk on water? | back 20 The spider is supported on the surface of the water due to the water’s strong surface tension that forms a tight, invisible film underneath the spider’s legs. |
front 21 What color is a positive test for proteins? | back 21 Purple |
front 22 You are working at the food lab when your boss gives you an unknown carbohydrate. You test the substance with biuret and Benedict’s reagents. The biuret test is blue as well as the Benedict’s test. What does this tell you about the sample? | back 22 negative for both reducing sugars and protein |
front 23 Which form do potatoes predominantly store their carbohydrates as? | back 23 Starch |
front 24 Give an example of form (structure) and function in biology. (I will accept examples used in lecture, or any example you may know from prior knowledge.) | back 24 Galapagos finches and their beaks. Hummingbird beaks. Gills on fish. Expansion of water molecules during freezing. Electronegativity of oxygen. Multiple examples can be used... structure and function are EVERYWHERE! |
front 25 Organisms are classified using taxonomic hierarchy. What two least inclusive levels are used universally to identify living organisms? | back 25 Genus and Species. |
front 26 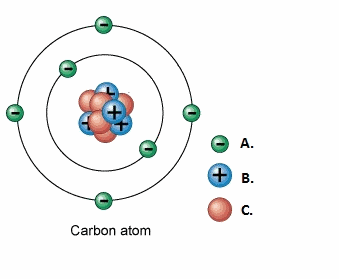 Label the following: | back 26 A.1.Electron B.2.Proton C.3.Neutron |
front 27 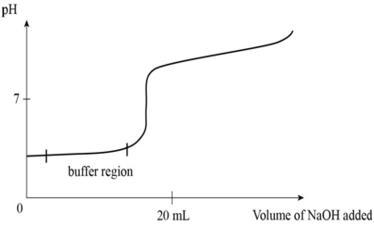 Is the following graph an example of a "good buffer"? Explain. | back 27 Yes. The pH is maintained at the buffer region, only with increased addition of NaOH does the buffer reach capacity and yield to change. |
front 28 Describe the difference between cohesion and adhesion. | back 28 Cohesion= water's affinity for itself. Adhesion= water's affinity for others (polar). |
front 29 ________ cells lack a membrane-enclosed nucleus. | back 29 Prokaryotic |
front 30 A bacterial cell's DNA is found in its | back 30 nucleoid region |
front 31 The membranous compartmentalization of a cell | back 31 allows different chemical conditions to be maintained in different parts of the cell. |
front 32 All of the following are part of a prokaryotic cell except | back 32 an endoplasmic reticulum |
front 33 Large numbers of ribosomes are present in cells that specialize in producing which of the following molecules? | back 33 proteins |
front 34 The liver is involved in detoxification of many poisons and drugs. Which of the following structures is primarily involved in this process and therefore abundant in liver cells? | back 34 smooth ER |
front 35 Tay-Sachs disease is a human genetic abnormality that results in cells accumulating and becoming clogged with very large, complex, undigested lipids. Which cellular organelle must be involved in this condition? | back 35 the lysosome |
front 36 In a plant cell, DNA may be found | back 36 in the nucleus, mitochondria, and chloroplasts. |
front 37 Unlike animal cells, plant cells have ________ and ________. Unlike plant cells, animal cells have ________. | back 37 chloroplasts . . . cell walls . . . centrioles |
front 38 Motor proteins provide for molecular motion in cells by interacting with what types of cellular structures? | back 38 cytoskeletal structures |
front 39 The fluid mosaic model describes the plasma membrane as consisting of | back 39 diverse proteins embedded in a phospholipid bilayer. |
front 40 In order for a protein to be an integral membrane protein it would have to be | back 40 amphipathic, with at least one hydrophobic region. |
front 41 What kinds of molecules pass through a cell membrane most easily? | back 41 small and hydrophobic |
front 42 Diffusion does not require the cell to expend ATP. Therefore, diffusion is considered a type of | back 42 passive transport |
front 43 Which of the following is a characteristic feature of a carrier protein in a plasma membrane? | back 43 It exhibits a specificity for a particular type of molecule. |
front 44 Which of the following statements correctly describes the normal tonicity conditions for typical plant and animal cells? | back 44 The animal cell is in an isotonic solution, and the plant cell is in a hypotonic solution |
front 45 Some protozoans have special organelles called contractile vacuoles that continually eliminate excess water from the cell. The presence of these organelles tells you that the environment | back 45 is hypotonic to the protozoan. |
front 46 You are adrift in the Atlantic Ocean, and, being thirsty, drink the surrounding seawater. As a result, | back 46 you dehydrate yourself. |
front 47 Which of the following processes can move a solute against its concentration gradient? | back 47 active transport |
front 48 The process of a white blood cell engulfing a bacterium is | back 48 phagocytosis. |
front 49 According to ________, energy cannot be created or destroyed. | back 49 the first law of thermodynamics |
front 50 Which of the following processes is endergonic? | back 50 the synthesis of glucose from carbon dioxide and water |
front 51 When a cell uses chemical energy to perform work, it uses the energy released from a(n) ________ reaction to drive a(n) ________ reaction. | back 51 exergonic . . . endergonic |
front 52 Which term most precisely describes the cellular process of breaking down large molecules into smaller ones? | back 52 catabolism |
front 53 Which of the following is an example of potential rather than kinetic energy? | back 53 a molecule of glucose |
front 54 When an enzyme catalyzes a reaction, | back 54 it lowers the activation energy of the reaction. |
front 55 The active site of an enzyme is | back 55 the region of an enzyme that attaches to a substrate. |
front 56 According to the induced fit hypothesis of enzyme catalysis, which of the following is correct? | back 56 The binding of the substrate changes the shape of the enzyme's active site. |
front 57 How does a noncompetitive inhibitor decrease the rate of an enzyme reaction? | back 57 by changing the shape of the enzyme's active site |
front 58 If an enzyme in solution is saturated with substrate, the most effective way to obtain a faster yield of products is to | back 58 add more of the enzyme. |
front 59 What is the magnification of the oculars (eyepieces) of the compound light microscope you used in the lab? | back 59 10x |
front 60 How does the field of view change as you move from the scanning to low power and then high power objectives? | back 60 The field of view decreases. |
front 61 Every plant cell contains chloroplasts, even if it is not directly involved in the process of photosynthesis. | back 61 False |
front 62 Samantha is working with a slide of bacteria and knows that she will need to use the high-power objective to see them clearly. She puts the slide on the microscope, centers it, rotates the high-power objective into place, and uses the coarse-adjustment knob to focus the image. What (if anything), has Samantha done wrong, and what could be the consequences of her actions? | back 62 Samantha should never use the coarse adjustment knob to focus with the high-power objective since the working distance is very small and this could damage both the lens and the slide. |
front 63 What were the results of the diffusion and osmosis experiment? | back 63 The glucose diffused outward from the bag, and the iodine diffused into the bag reacting with starch. |
front 64 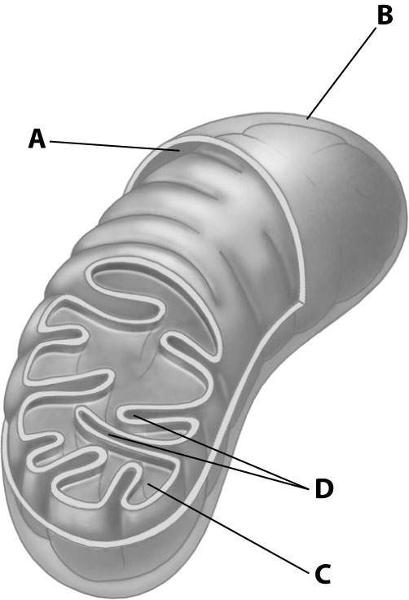 Which part of the mitochondrion shown enhances its ability to produce ATP by increasing the surface area of a mitochondrial membrane? | back 64 Structure D |
front 65 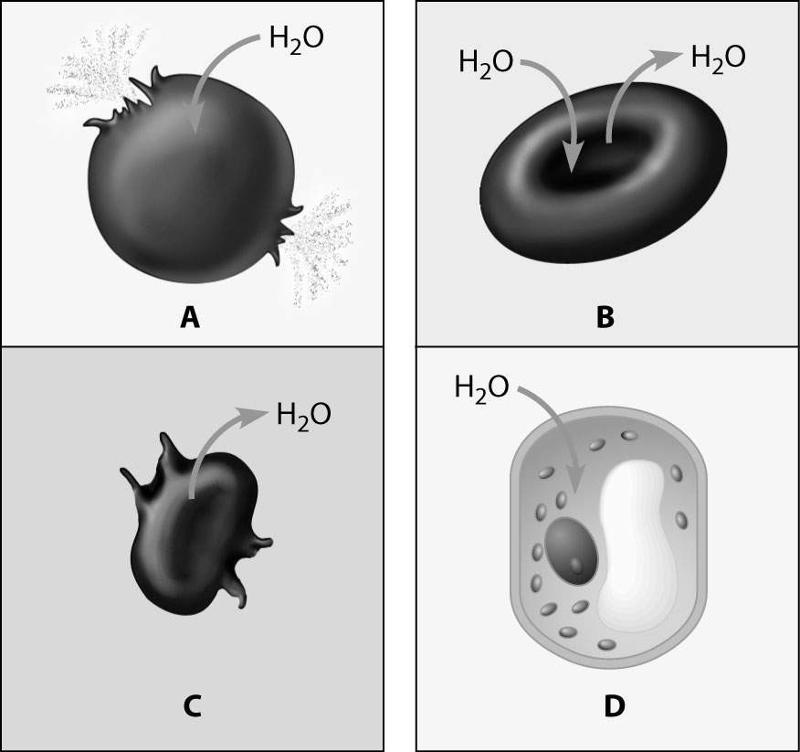 Which figure depicts an animal cell placed in a solution hypotonic to the cell? | back 65 Cell A |
front 66 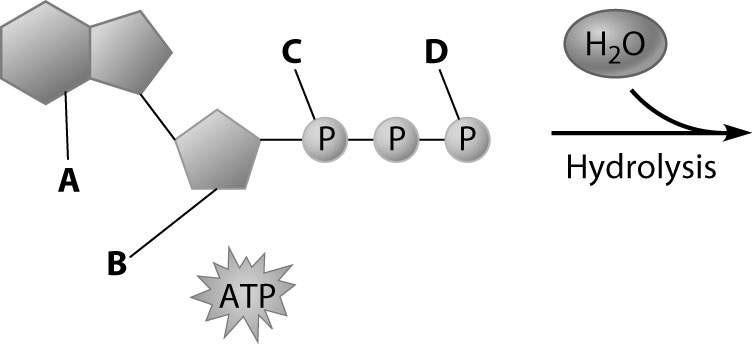 Which part of the ATP molecule breaks free of the rest when an ATP molecule is used for energy? | back 66 Part D |
front 67
Americans spend up to $100 billion annually for bottled water (41 billion gallons). The only beverages with higher sales are carbonated soft drinks. Recent news stories have highlighted the fact that most bottled water comes from municipal water supplies (the same source as your tap water), although it may undergo an extra purification step called reverse osmosis. Imagine two tanks that are separated by a membrane that's permeable to water, but not to the dissolved minerals present in the water. Tank A contains tap water and Tank B contains the purified water. Under normal conditions, the purified water would cross the membrane to dilute the more concentrated tap water solution. In the reverse osmosis process, pressure is applied to the tap water tank to force the water molecules across the membrane into the pure water tank. After the reverse osmosis system has been operating for 30 minutes, the solution in Tank A would | back 67 be hypertonic to Tank B. |
front 68 If you shut the system off and pressure was no longer applied to Tank A, you would expect | back 68 the water to reverse flow from Tank B to Tank A |
front 69 Which of the following statements regarding photosynthesis and cellular respiration is true? | back 69 Photosynthesis occurs in chloroplasts, and cellular respiration occurs in mitochondria. |
front 70 What is the term for metabolic pathways that release stored energy by breaking down complex molecules? | back 70 catabolic pathways |
front 71 The molecule that functions as the reducing agent (electron donor) in a redox or oxidation-reduction reaction | back 71 loses electrons and loses potential energy. |
front 72 How do cells capture the energy released by cellular respiration? | back 72 They produce ATP. |
front 73 Respiration ________, and cellular respiration ________. | back 73 is gas exchange . . . produces ATP |
front 74 Which of the following are products of cellular respiration? | back 74 energy to make ATP and carbon dioxide |
front 75 The overall equation for the cellular respiration of glucose is | back 75 C6H12O6 + 6 O2 → 6 CO2 + 6 H2O + energy. |
front 76 Oxidation is the ________, and reduction is the ________. | back 76 loss of electrons . . . gain of electrons |
front 77 Which of the following options lists the stages in cellular respiration in the correct order? | back 77 glycolysis, the citric acid cycle, and oxidative phosphorylation |
front 78 Which of the following metabolic pathways is common in aerobic and anaerobic metabolism? | back 78 glycolysis |
front 79 Where does glycolysis take place in eukaryotic cells? | back 79 cytosol |
front 80 Why is glycolysis described as having an investment phase and a payoff phase? | back 80 It uses stored ATP and then forms a net increase in ATP. |
front 81 Pyruvate | back 81 forms at the end of glycolysis. |
front 82 After glycolysis but before the citric acid cycle, | back 82 pyruvate is oxidized. |
front 83 In the absence of oxygen, yeast cells can obtain energy by fermentation, resulting in the production of | back 83 ATP, CO2, and ethanol (ethyl alcohol). |
front 84 The molecule that functions as the reducing agent (electron donor) in a redox or oxidation-reduction reaction | back 84 loses electrons and loses potential energy. |
front 85 What is the likely origin of chloroplasts? | back 85 photosynthetic prokaryotes that lived inside eukaryotic cells |
front 86 In most green plants, chloroplasts are | back 86 concentrated in a zone of leaf tissue called the mesophyll. |
front 87 CO2 enters and O2 escapes from a leaf via | back 87 stomata. |
front 88 In the chloroplast, sugars are made in a compartment that is filled with a thick fluid called the | back 88 stroma. |
front 89 Chloroplasts contain disklike membranous sacs arranged in stacks called | back 89 grana. |
front 90 Where is chlorophyll found in a plant cell? | back 90 thylakoid membranes |
front 91 The oxygen released into the air as a product of photosynthesis comes from | back 91 water. |
front 92 What is the source of energy that provides the boost for electrons during photosynthesis? | back 92 light |
front 93 The light reactions occur in the ________, while the Calvin cycle occurs in the ________. | back 93 thylakoid membranes . . . stroma |
front 94 Carbon fixation | back 94 occurs when carbon atoms from CO2 are incorporated into an organic molecule. |
front 95 Which of the following are produced during the light reactions of photosynthesis? | back 95 ATP, NADPH, O2 |
front 96 Which of the following are produced during the Calvin cycle? | back 96 glucose, ADP, NADP+ |
front 97 Which of the following colors contributes the least energy to photosynthesis? | back 97 green |
front 98 A packet of light energy is called a | back 98 photon. |
front 99 Briefly describe the relationship between cell respiration and photosynthesis. | back 99 The products of cellular respirations are the reactants of photosynthesis. Both are intrinsically linked within the life cycle of our ecosystem. |
front 100 Your friend has completed a day of heavy weight lifting at the local gym that was interrupted by moments of muscle burning relieved by rest. They are curious as to what type of biological mechanism is at work. Please briefly explain what is occurring at the cellular level and how it relates to your friends "muscle burning". | back 100 Your friend is experiencing a build up of lactic acid. This occurs in muscle cells because they are facultative anaerobes. When oxygen is present, ATP is produced. When oxygen is not present, muscle cells with enter into fermentation producing lactic acid. |
front 101 Which of the following statements regarding prokaryotes is false? | back 101 Prokaryotic chromosomes are more complex than those of eukaryotes. |
front 102 Eukaryotic chromosomes differ from prokaryotic chromosomes in that they | back 102 are housed in a membrane-enclosed nucleus. |
front 103 Prior to mitosis, each chromosome of a eukaryotic cell consists of a pair of identical structures called | back 103 sister chromatids. |
front 104 Eukaryotic cells spend most of their cell cycle in which phase? | back 104 interphase |
front 105 The genetic material is duplicated during | back 105 the S phase. |
front 106 The process by which the cytoplasm of a eukaryotic cell divides to produce two cells is called | back 106 cytokinesis |
front 107 The process by which the cytoplasm of a eukaryotic cell divides to produce two cells is called | back 107 metaphase |
front 108 At the start of mitotic anaphas | back 108 the centromeres of each chromosome come apart. |
front 109 During which phase of mitosis does the nuclear envelope re-form? | back 109 telophase |
front 110 As a patch of scraped skin heals, the cells fill in the injured area but do not grow beyond that. This is an example of | back 110 density-dependent inhibition. |
front 111 Mature human nerve cells and muscle cells | back 111 are permanently in a state of nondivision. |
front 112 During which stage of meiosis do synapsis and crossing over occur? | back 112 prophase I |
front 113 Independent orientation of chromosomes at metaphase I results in an increase in the number of | back 113 possible combinations of characteristics. |
front 114 Karyotyping | back 114 can reveal alterations in chromosome number. |
front 115 Nondisjunction occurs when | back 115 members of a chromosome pair fail to separate. |
front 116 Mendel conducted his most memorable experiments on | back 116 peas |
front 117 A monohybrid cross is | back 117 a breeding experiment in which the parental varieties differ in only one character. |
front 118 All the offspring of a cross between a black-eyed mendelien and an orange-eyed mendelien have black eyes. This means that the allele for black eyes is ________ the allele for orange eyes. | back 118 dominant to |
front 119 The alleles of a gene are found at ________ chromosomes. | back 119 the same locus on homologous |
front 120 The phenotypic ratio resulting from a dihybrid cross showing independent assortment is expected to be | back 120 9:3:3:1. |
front 121 A testcross is | back 121 a mating between an individual of unknown genotype and an individual homozygous recessive for the trait of interest. |
front 122 All the offspring of a cross between a red-flowered plant and a white-flowered plant have pink flowers. This means that the allele for red flowers is ________ to the allele for white flowers. | back 122 incompletely dominant |
front 123 What is the normal complement of sex chromosomes in a human male? | back 123 one X chromosome and one Y chromosome |
front 124 Recessive X-linked traits are more likely to be expressed in a male fruit fly than a female fruit fly because | back 124 the male's phenotype results entirely from his single X-linked gene. |
front 125 Which of the following variations of the sentence "Where is the cat" is most like a chromosomal deletion? | back 125 Where is cat? |
front 126 If a chromosome fragment breaks off and then reattaches to the original chromosome, but in the reverse direction, the resulting chromosomal abnormality is called a(n) | back 126 inversion. |
front 127 Asexual reproduction requires ________ individual(s). | back 127 1 |
front 128 Which of the following statements regarding genetic diversity is false? | back 128 Genetic diversity is enhanced by mitosis. |
front 129 Cancer is not usually inherited because | back 129 Cancer is not usually inherited because |
front 130 The individual features of all organisms are the result of | back 130 genetics and the environment. |