Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Chapter 7-10 Biology Final
front 1 Some regions of the plasma membrane, called lipid rafts, have a
higher concentration of cholesterol molecules. As a result, these
lipid rafts | back 1 Answer : B |
front 2 Which of the following types of molecules are the major structural
components of the cell membrane? | back 2 Answer : C |
front 3 When biological membranes are frozen and then fractured, they tend to
break along the middle of the bilayer. The best explanation for this
is that | back 3 Answer : E |
front 4 The presence of cholesterol in the plasma membranes of some animals
| back 4 Answer : A |
front 5 According to the fluid mosaic model of cell membranes, which of the
following is a true statement about membrane phospholipids? | back 5 Answer : A |
front 6 Which of the following is one of the ways that the membranes of
winter wheat are able to remain fluid when it is extremely cold?
B) by increasing the percentage of unsaturated phospholipids in the membrane C) by decreasing the number of hydrophobic proteins in the membrane D) by cotransport of glucose and hydrogen E) by using active transport | back 6 Answer : B |
front 7 In order for a protein to be an integral membrane protein it would
have to be | back 7 Answer : C |
front 8 Which of the following is a reasonable explanation for why
unsaturated fatty acids help keep any membrane more fluid at lower
temperatures? | back 8 Answer : A |
front 9 The primary function of polysaccharides attached to the glycoproteins
and glycolipids of animal cell membranes is | back 9 Answer : E |
front 10 Which of these are not embedded in the hydrophobic portion of the
lipid bilayer at all? | back 10 Answer : C |
front 11 The cell membranes of Antarctic ice fish might have which of the
following adaptations? | back 11 Answer : C |
front 12 The formulation of a model for a structure or for a process serves
which of the following purposes? | back 12 Answer : B |
front 13 Cell membranes are asymmetrical. Which of the following is the most
likely explanation? | back 13 Answer : C |
front 14 Why are lipids and proteins free to move laterally in membranes?
| back 14 Answer : D |
front 15 What kinds of molecules pass through a cell membrane most easily?
| back 15 Answer : B |
front 16 Which of the following is a characteristic feature of a carrier
protein in a plasma membrane? | back 16 Answer : B |
front 17 Which of the following statements is correct about diffusion?
| back 17 Answer : C |
front 18 Water passes quickly through cell membranes because | back 18 Answer : E |
front 19 Celery stalks that are immersed in fresh water for several hours
become stiff and hard. Similar stalks left in a 0.15 M salt solution
become limp and soft. From this we can deduce that the cells of the
celery stalks are | back 19 Answer : C |
front 20 Mammalian blood contains the equivalent of 0.15 M NaCl. Seawater
contains the equivalent of 0.45 M NaCl. What will happen if red blood
cells are transferred to seawater? | back 20 Answer : A |
front 21 When a plant cell, such as one from a peony stem, is submerged in a
very hypotonic solution, what is likely to occur? | back 21 Answer : E |
front 22 Which of the following membrane activities require energy from ATP
hydrolysis? | back 22 Answer : C |
front 23 The phosphate transport system in bacteria imports phosphate into the
cell even when the concentration of phosphate outside the cell is much
lower than the cytoplasmic phosphate concentration. Phosphate import
depends on a pH gradient across the membrane–more acidic outside the
cell than inside the cell. Phosphate transport is an example of
| back 23 Answer : E |
front 24 Glucose diffuses slowly through artificial phospholipid bilayers. The
cells lining the small intestine, however, rapidly move large
quantities of glucose from the glucose-rich food into their
glucose-poor cytoplasm. Using this information, which transport
mechanism is most probably functioning in the intestinal cells?
| back 24 Answer : E |
front 25 What is the voltage across a membrane called? | back 25 Answer : C |
front 26 The sodium-potassium pump is called an electrogenic pump because it
| back 26 Answer : C |
front 27 Which of the following would increase the electrochemical potential
across a membrane? | back 27 Answer : A |
front 28 White blood cells engulf bacteria through what process? | back 28 Answer : B |
front 29 Familial hypercholesterolemia is characterized by which of the
following? | back 29 Answer : A |
front 30 The difference between pinocytosis and receptor-mediated endocytosis
is that | back 30 Answer : C |
front 31 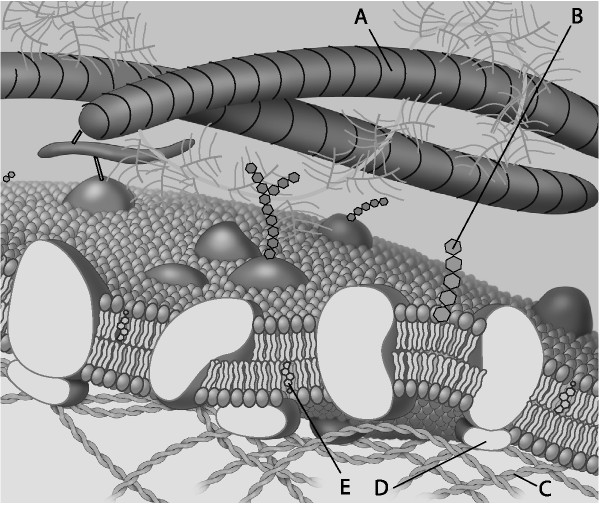 Which component is the peripheral protein? | back 31 Answer : D |
front 32 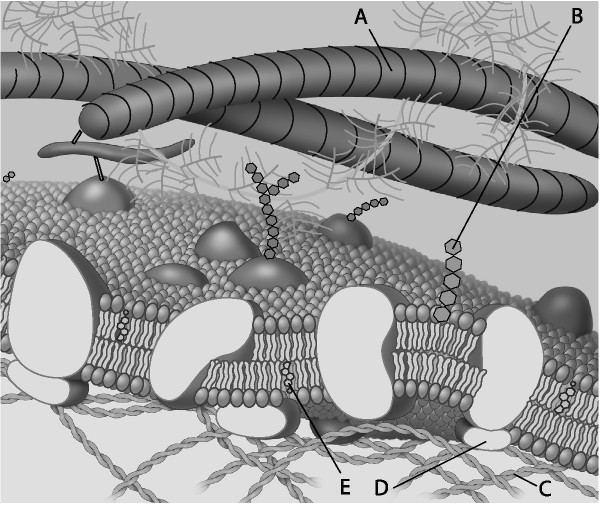 Which component is cholesterol? | back 32 Answer : E |
front 33 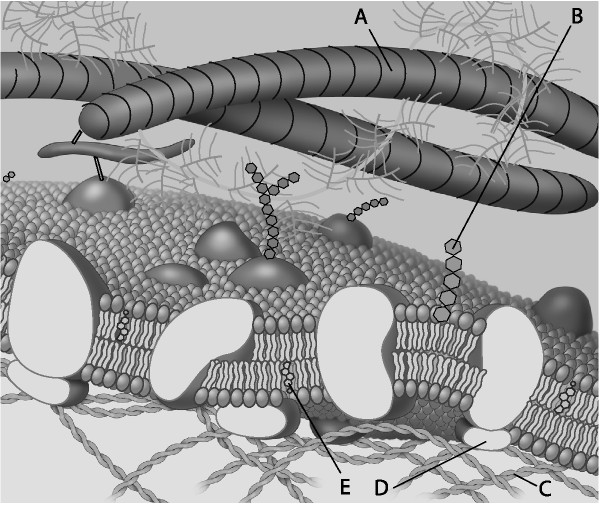 Which component is the fiber of the extracellular matrix? | back 33 Answer : A |
front 34 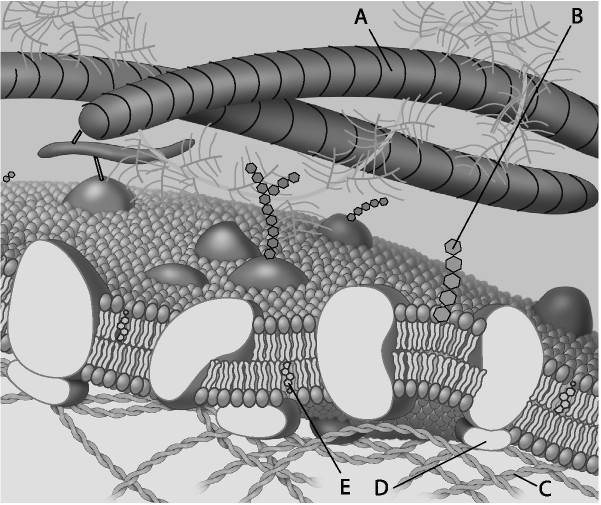 Which component is a microfilament of the cytoskeleton? | back 34 Answer : C |
front 35 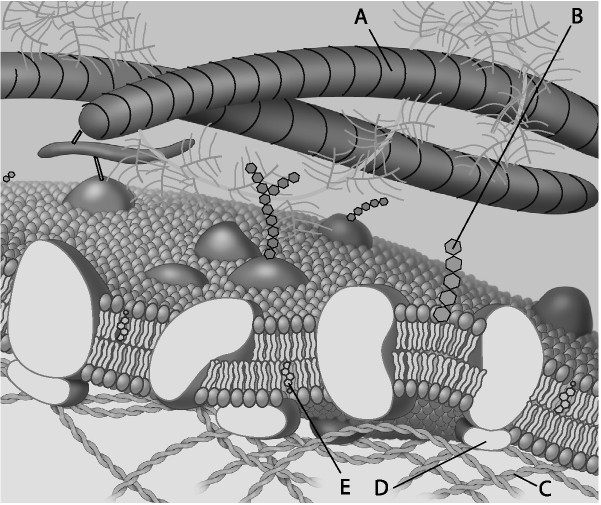 Which component is a glycolipid? | back 35 Answer : B |
front 36 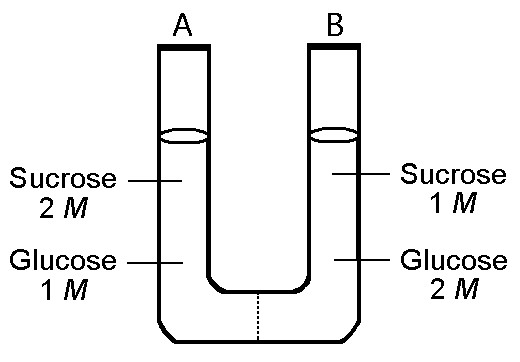 The solutions in the two arms of this U-tube are separated by a
membrane that is permeable to water and glucose but not to sucrose.
Side A is half-filled with a solution of 2 M sucrose and 1 M glucose.
Side B is half-filled with 1 M sucrose and 2 M glucose. Initially, the
liquid levels on both sides are equal. | back 36 Answer : C |
front 37 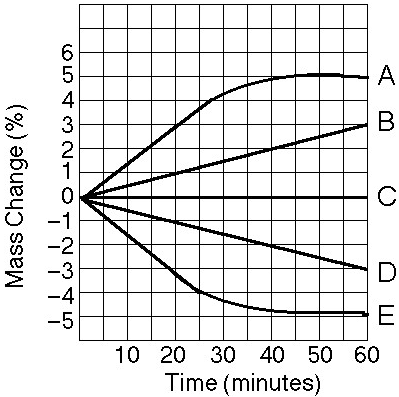 Five dialysis bags, constructed from a semipermeable membrane that is
impermeable to sucrose, were filled with various concentrations of
sucrose and then placed in separate beakers containing an initial
concentration of 0.6 M sucrose solution. At 10-minute intervals, the
bags were massed (weighed) and the percent change in mass of each bag
was graphed. | back 37 Answer : C |
front 38 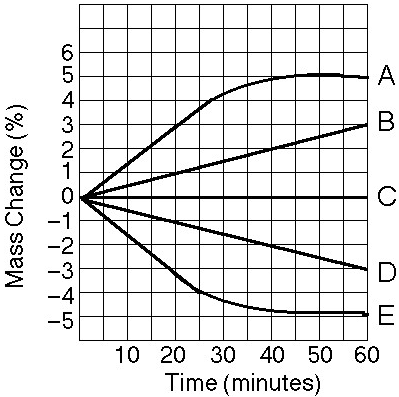 Which line in the graph represents the bag with the highest initial
concentration of sucrose? | back 38 Answer : A |
front 39 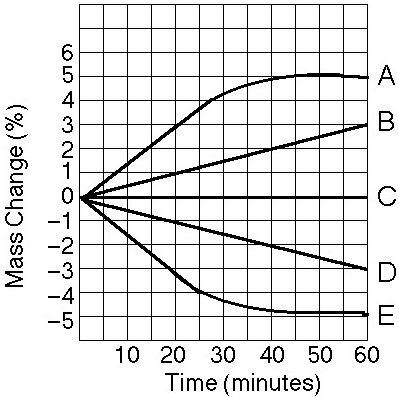 Which line or lines in the graph represent(s) bags that contain a
solution that is hypertonic at 50 minutes? | back 39 Answer : B |
front 40 A patient has had a serious accident and lost a lot of blood. In an
attempt to replenish body fluids, distilled water–equal to the volume
of blood lost–is transferred directly into one of his veins. What will
be the most probable result of this transfusion? | back 40 Answer : C |
front 41 Which term most precisely describes the cellular process of breaking
down large molecules into smaller ones? | back 41 Answer : E |
front 42 Which of the following is (are) true for anabolic pathways? | back 42 Answer : C |
front 43 Whenever energy is transformed, there is always an increase in the
| back 43 Answer : D |
front 44 Which of the following types of reactions would decrease the entropy
within a cell? | back 44 Answer : A |
front 45 Which of the following is true for all exergonic reactions? | back 45 Answer : B |
front 46 Why is ATP an important molecule in metabolism? | back 46 Answer : B |
front 47 Which of the following is most similar in structure to ATP? | back 47 Answer : C |
front 48 When chemical, transport, or mechanical work is done by an organism,
what happens to the heat generated? | back 48 Answer : D |
front 49 What is the difference (if any) between the structure of ATP and the
structure of the precursor of the A nucleotide in RNA? | back 49 Answer : E |
front 50 Which of the following statements is true about enzyme-catalyzed
reactions? | back 50 Answer : A |
front 51 Reactants capable of interacting to form products in a chemical
reaction must first overcome a thermodynamic barrier known as the
reaction's | back 51 Answer : B |
front 52 A solution of starch at room temperature does not readily decompose
to form a solution of simple sugars because | back 52 Answer : C |
front 53 Which of the following statements regarding enzymes is true? | back 53 Answer : B |
front 54 The active site of an enzyme is the region that | back 54 Answer : B |
front 55 According to the induced fit hypothesis of enzyme catalysis, which of
the following is correct? | back 55 Answer: D |
front 56 Mutations that result in single amino acid substitutions in an enzyme
| back 56 Answer : D |
front 57 Increasing the substrate concentration in an enzymatic reaction could
overcome which of the following? | back 57 Answer : C |
front 58 When you have a severe fever, what grave consequence may occur if the
fever is not controlled? | back 58 Answer : C |
front 59 How does a noncompetitive inhibitor decrease the rate of an enzyme
reaction? | back 59 Answer : B |
front 60 The mechanism in which the end product of a metabolic pathway
inhibits an earlier step in the pathway is most precisely described as
| back 60 Answer : A |
front 61 Besides turning enzymes on or off, what other means does a cell use
to control enzymatic activity? | back 61 Answer : B |
front 62 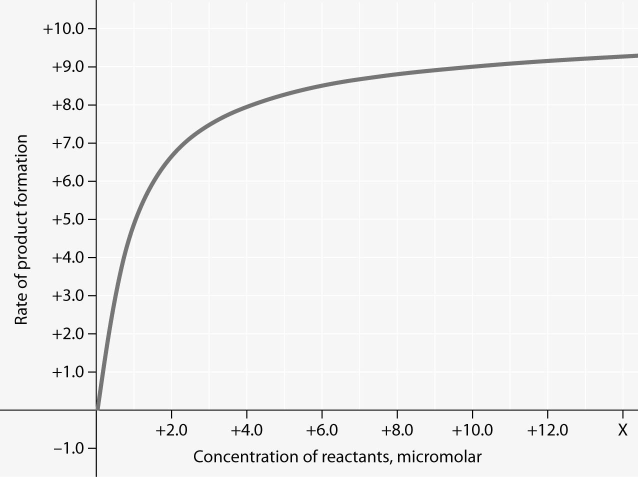 For the enzyme-catalyzed reaction shown in the figure, which of these
treatments will cause the greatest increase in the rate of the
reaction, if the initial reactant concentration is 1.0 micromolar?
| back 62 Answer : D |
front 63 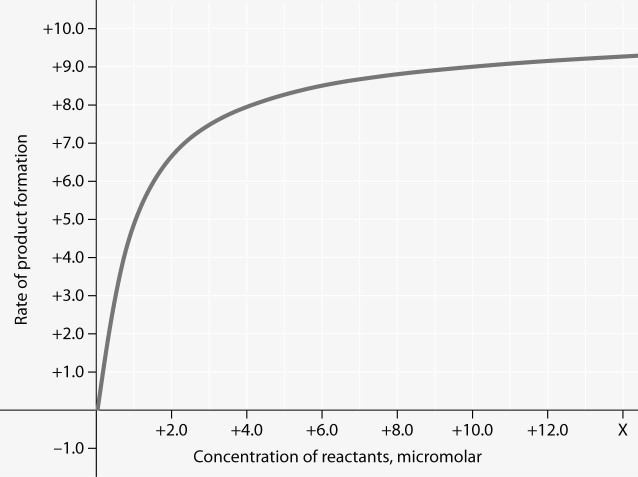 In the figure, why does the reaction rate plateau at higher reactant
concentrations? | back 63 Answer : B |
front 64 What is the term for metabolic pathways that release stored energy by
breaking down complex molecules? | back 64 Answer : B |
front 65 When electrons move closer to a more electronegative atom, what
happens? | back 65 Answer : A |
front 66 Which of the following statements describes the results of this
reaction? | back 66 Answer : A |
front 67 When a glucose molecule loses a hydrogen atom as the result of an
oxidation-reduction reaction, the molecule becomes | back 67 Answer : C |
front 68 When a molecule of NAD⁺ (nicotinamide adenine dinucleotide) gains a
hydrogen atom (not a proton), the molecule becomes | back 68 Answer : D |
front 69 Where does glycolysis take place in eukaryotic cells? | back 69 Answer : E |
front 70 The oxygen consumed during cellular respiration is involved directly
in which process or event? | back 70 Answer : B |
front 71 Which process in eukaryotic cells will proceed normally whether
oxygen (O₂) is present or absent? | back 71 Answer : B |
front 72 Substrate-level phosphorylation accounts for approximately what
percentage of the ATP formed by the reactions of glycolysis? | back 72 Answer : E |
front 73 In addition to ATP, what are the end products of glycolysis? | back 73 Answer : C |
front 74 In glycolysis, for each molecule of glucose oxidized to pyruvate
| back 74 Answer : B |
front 75 Why is glycolysis described as having an investment phase and a
payoff phase? | back 75 Answer : E |
front 76 Which of the following intermediary metabolites enters the citric
acid cycle and is formed, in part, by the removal of a carbon (CO₂)
from one molecule of pyruvate? | back 76 Answer : D |
front 77 How many carbon atoms are fed into the citric acid cycle as a result
of the oxidation of one molecule of pyruvate? | back 77 Answer : A |
front 78 Carbon dioxide (CO₂) is released during which of the following stages
of cellular respiration? | back 78 Answer : B |
front 79 During aerobic respiration, electrons travel downhill in which
sequence? | back 79 Answer : B |
front 80 What fraction of the carbon dioxide exhaled by animals is generated
by the reactions of the citric acid cycle, if glucose is the sole
energy source? | back 80 Answer : D |
front 81 Where are the proteins of the electron transport chain located?
| back 81 Answer : C |
front 82 In cellular respiration, the energy for most ATP synthesis is
supplied by | back 82 Answer : B |
front 83 The primary role of oxygen in cellular respiration is to | back 83 Answer : B |
front 84 During aerobic respiration, H₂O is formed. Where does the oxygen atom
for the formation of the water come from? | back 84 Answer : C |
front 85 Energy released by the electron transport chain is used to pump H⁺
into which location in eukaryotic cells? | back 85 Answer : D |
front 86 Where is ATP synthase located in the mitochondrion? | back 86 Answer : D |
front 87 How many oxygen molecules (O₂) are required each time a molecule of
glucose (C₆H₁₂O₆) is completely oxidized to carbon dioxide and water
via aerobic respiration,? | back 87 Answer : C |
front 88 Approximately how many molecules of ATP are produced from the
complete oxidation of two molecules of glucose (C₆H₁₂O₆) in aerobic
cellular respiration? | back 88 Answer : E |
front 89 If a cell is able to synthesize 30 ATP molecules for each molecule of
glucose completely oxidized by carbon dioxide and water, how many ATP
molecules can the cell synthesize for each molecule of pyruvate
oxidized to carbon dioxide and water? | back 89 Answer : C |
front 90 Which of the following normally occurs regardless of whether or not
oxygen (O₂) is present? | back 90 Answer : A |
front 91 The ATP made during fermentation is generated by which of the
following? | back 91 Answer : B |
front 92 In the absence of oxygen, yeast cells can obtain energy by
fermentation, resulting in the production of | back 92 Answer : A |
front 93 One function of both alcohol fermentation and lactic acid
fermentation is to | back 93 Answer : C |
front 94 An organism is discovered that thrives both in the presence and
absence of oxygen in the air. Curiously, the consumption of sugar
increases as oxygen is removed from the organism's environment, even
though the organism does not gain much weight. This organism | back 94 Answer : E |
front 95 When an individual is exercising heavily and when the muscle becomes
oxygen-deprived, muscle cells convert pyruvate to lactate. What
happens to the lactate in skeletal muscle cells? | back 95 Answer : C |
front 96 Phosphofructokinase is an allosteric enzyme that catalyzes the
conversion of fructose 6-phosphate to fructose 1,6-bisphosphate, an
early step of glycolysis. In the presence of oxygen, an increase in
the amount of ATP in a cell would be expected to | back 96 Answer : A |
front 97 Which of the following are products of the light reactions of
photosynthesis that are utilized in the Calvin cycle? | back 97 Answer : E |
front 98 Photosynthesis is not responsible for | back 98 Answer : E |
front 99 Where does the Calvin cycle take place? | back 99 Answer : A |
front 100 In any ecosystem, terrestrial or aquatic, what group(s) is (are)
always necessary? | back 100 Answer : D |
front 101 When oxygen is released as a result of photosynthesis, it is a direct
by-product of | back 101 Answer : B |
front 102 Which of the events listed below occurs in the light reactions of
photosynthesis? | back 102 Answer : E |
front 103 Reduction of NADP⁺ occurs during | back 103 Answer : A |
front 104 The splitting of carbon dioxide to form oxygen gas and carbon
compounds occurs during | back 104 Answer : D |
front 105 Generation of proton gradients across membranes occurs during
| back 105 Answer : C |
front 106 What is the relationship between wavelength of light and the quantity
of energy per photon? | back 106 Answer : B |
front 107 The reactions that produce molecular oxygen (O₂) take place in
| back 107 Answer : A |
front 108 What is the primary function of the Calvin cycle? | back 108 Answer : E |
front 109 The NADPH required for the Calvin cycle comes from | back 109 Answer : A |
front 110 Reactions that require CO₂ take place in | back 110 Answer : B |
front 111 Which of the following statements best represents the relationships
between the light reactions and the Calvin cycle? | back 111 Answer : A |
front 112 In the process of carbon fixation, RuBP attaches a CO₂ to produce a
six-carbon molecule, which is then split to produce two molecules of
3-phosphoglycerate. After phosphorylation and reduction produces
glyceraldehyde 3-phosphate (G3P), what more needs to happen to
complete the Calvin cycle? | back 112 Answer : D |
front 113 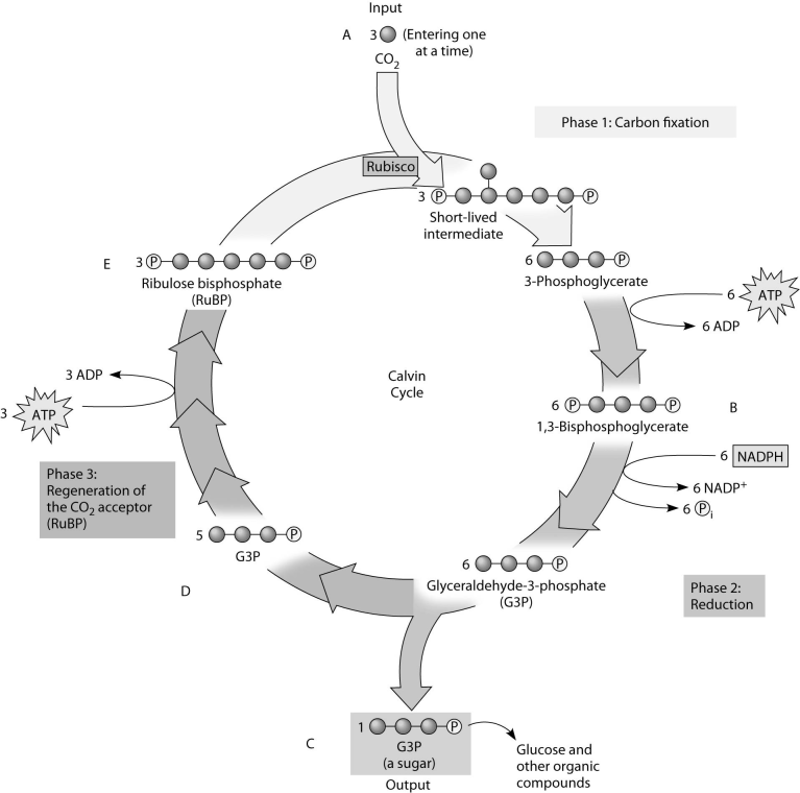 Which molecule(s) of the Calvin cycle is (are) also found in
glycolysis? | back 113 Answer : D |
front 114 A gardener is concerned that her greenhouse is getting too hot from
too much light, and seeks to shade her plants with colored translucent
plastic sheets. What color should she use to reduce overall light
energy, but still maximize plant growth? | back 114 Answer : B |
front 115 Theodor W. Engelmann illuminated a filament of algae with light that
passed through a prism, thus exposing different segments of algae to
different wavelengths of light. He added aerobic bacteria and then
noted in which areas the bacteria congregated. He noted that the
largest groups were found in the areas illuminated by the red and blue
light. | back 115 Answer : C |
front 116 Theodor W. Engelmann illuminated a filament of algae with light that
passed through a prism, thus exposing different segments of algae to
different wavelengths of light. He added aerobic bacteria and then
noted in which areas the bacteria congregated. He noted that the
largest groups were found in the areas illuminated by the red and blue
light. | back 116 Answer : D |
front 117 A spaceship is designed to support animal life for a multiyear voyage
to the outer planets of the solar system. Plants will be grown to
provide oxygen and to recycle carbon dioxide. | back 117 Answer : C |
front 118 A spaceship is designed to support animal life for a multiyear voyage
to the outer planets of the solar system. Plants will be grown to
provide oxygen and to recycle carbon dioxide. | back 118 Answer : A |