Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Bio Final
front 1 Which of the following is a FALSE statement concerning amino groups? Amino groups ______. A. Contain Nitrogen B. Are basic with respect to pH C. Are found in amino acids D. Are nonpolar | back 1 D. Are nonpolar |
front 2 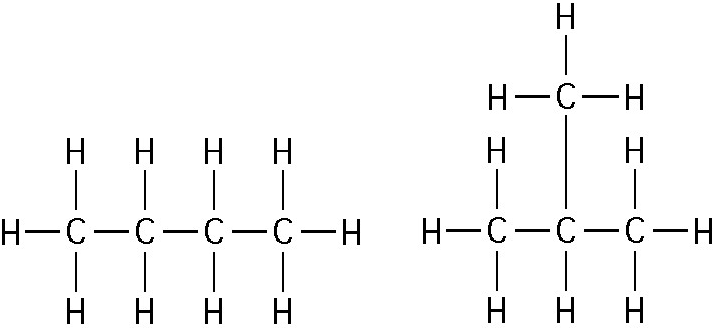 The two molecules shown in the figure below are best described as ______. A. Structural isomers B. Cis-trans isomers C. Chain length isomers D. Enantiomers | back 2 A. Structural isomers |
front 3 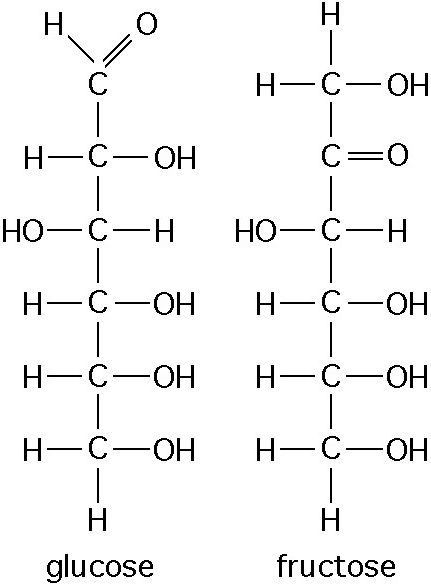 The figure above shows the structure of glucose and fructose. These two molecules differ in the _____. A. Number of carbon, hydrogen, and oxygen atoms B. Types of carbon, hydrogen, and oxygen atoms C. Arrangement of carbon, hydrogen, and oxygen atoms D. Number of oxygen atoms joined to carbon atoms by double covalent bonds | back 3 C. Arrangement of carbon, hydrogen, and oxygen atoms |
front 4 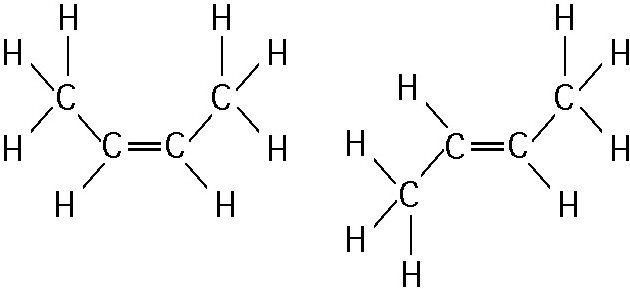 The two molecules shown in the figure below are best described as _____. A. Enantiomers B. Cis-trans isomers C. Structural isomers D. Radioactive isotpes | back 4 B. Cis-trans isomers |
front 5 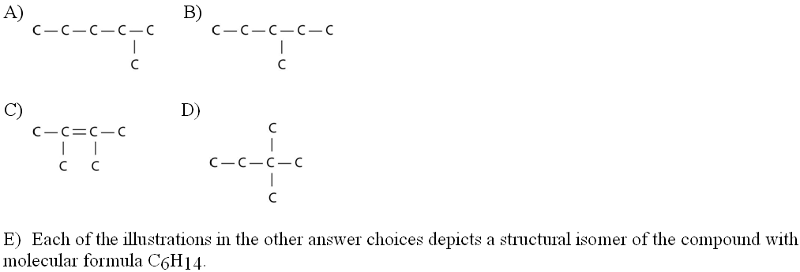 Which of the following illustrations is NOT a structural isomer of an organic compound with the molecular formula C6H14? For clarity, only the carbon skeletons are shown; hydrogen atoms that would be attached to the carbons have been omitted. | back 5 C. |
front 6 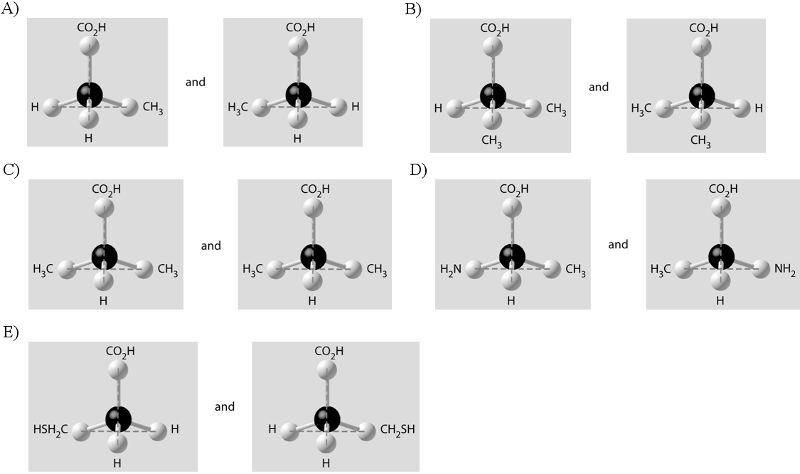 Which of the pairs of molecular structures shown below depict enantiomers (enantiomeric forms) of the same molecule? | back 6 D. |
front 7 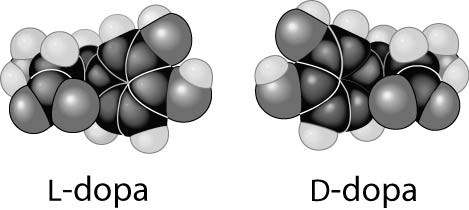 Thalidomide and L-dopa, shown below, are examples of pharmaceutical
drugs that occur as enantiomers, or molecules that | back 7 B. are mirror images of one another. |
front 8 A compound contains hydroxyl groups as its predominant functional group. Therefore, this compound _____. A. Should dissolve in a nonpolar solvent B. Lacks an asymmetric carbon and is probably fat or lipid C. Will not form hydrogen bonds with water D. Should dissolve in water | back 8 D. Should dissolve in water |
front 9 Which of the functional groups below act most like an acid in water? A. Hydroxyl B. Carbonyl C. Carboxyl D. Amino | back 9 C. Carboxyl |
front 10 Which of the two functional groups are always found in amino acids? A. Carbonyl and amino groups B. Amino and Sulfhydrl groups C. Carboxyl and amino groups D. Hydroxyl and carboxyl groups | back 10 C. Carboxyl and amino groups |
front 11 Amino acids are acids because they always posses which functional group? A. Phosphate B. Carboxyl C. Amino D. Carbonyl | back 11 B. Carboxyl |
front 12 A hydrocarbon skeleton is covalently boned to an amino group at one end and a carboxyl group at the other end. When placed in water this molecule would function ____. A. As an acid and a base B. Only as an acid because of the carboxyl group C. Only as a base because of the amino group D. As neither an acid nor a base | back 12 A. As an acid and a base |
front 13 Which chemical group can act as an acid? A. Carbonyl B. Amino C. Carboxyl D. Methyl | back 13 C. Carboxyl |
front 14 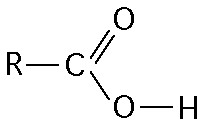 What is the name of the functional group shown in the figure above?
| back 14 D. Carboxyl |
front 15 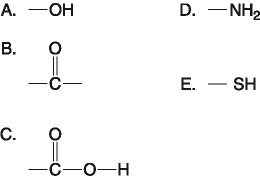 Which functional group shown above is characteristic of
alcohols? B. B C. C D. D | back 15 A. A |
front 16 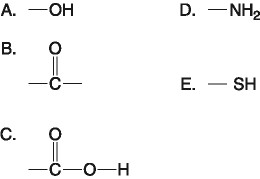 Which functional group(s) shown above is (are) present in all amino acids? A) A and B | back 16 E. C and D |
front 17 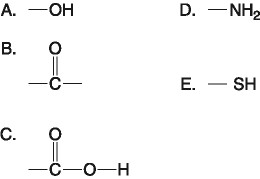 Which of the groups shown above is a functional group that helps stabilize proteins by forming covalent cross-links within or between protein molecules? A. A B. B C. C D. D E. E | back 17 E. E |
front 18 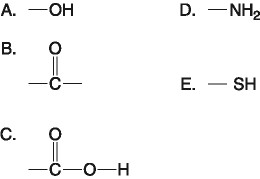 Which of the groups above is an acidic functional group that can dissociate and release H+ into a solution? A. A B. B C. C D. D E. E | back 18 C. C |
front 19 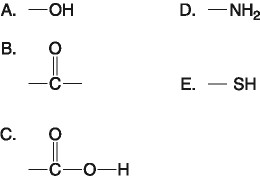 Which of the groups above is a basic functional group that can accept H+ and become positively charged? A. A B. B C. C D. D E. E | back 19 D. D |
front 20 Testosterone and estradiol are male and female sex hormones, respectively, in many vertebrates. In what way(s) do these molecules differ from each other? Testosterone and estradiol ______. A. have different functional groups attached to the same carbon skeleton B. are entantiomers of the same organic molecule C. are structural isomers but have the same molecular formula D. are cis-trans isomers but have the same molecular formula | back 20 A. have different functional groups attached to the same carbon skeleton |
front 21 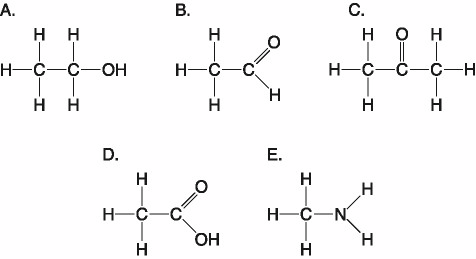 Which molecules shown above contain a carbonyl group? | back 21 B. B and C |
front 22 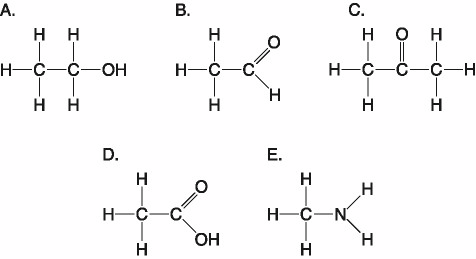 Which molecule shown above has a carbonyl functional group in the
form of a ketone? | back 22 C. C |
front 23 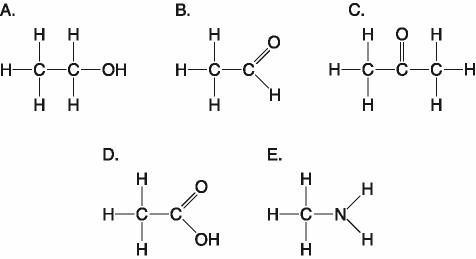 Which molecule shown above has a carbonyl functional group in the
form of an aldehyde? | back 23 B. B |
front 24 Stanley Miller's 1953 experiments supported the hypothesis that _____. A. life on Earth arose from simple inorganic molecules B. the conditions on early Earth were conductive to the origin of life C. life on Earth arose from simple organic molecules, with energy from lightning and volcanoes D. organic molecules can be synthesized abiotically under conditions that may have existed on early Earth | back 24 D. organic molecules can be synthesized abiotically under conditions that may have existed on early Earth |
front 25 When Stanley Miller applied heat and electrical sparks to a mixture of simple inorganic compounds such as methane, hydrogen gas, ammonia, and water vapor, what compounds were produced? A. mostly hydrocarbons B. only simple inorganic compounds C. simple organic compounds, amino acids, and hydrocarbons D. only simple organic compounds such as formaldehyde and cyanide | back 25 C. simple organic compounds. amino acids, and hydrocarbons |
front 26  How many neutrons are present in the nucleus of a phosphorus-32 (³²P)
atom (see the figure above)? | back 26 D. 17 |
front 27  How many electrons does an atom of sulfur have in its valence shell
(see the figure above)? | back 27 B. 6 |
front 28 Which of these is an example of inductive reasoning? A. If protists are all single-celled, then they are incapable of aggregating B. These organisms live in sunny regions. Therefore, they are using photosynthesis C. Hundreds of individuals of a species have been observed and all are photosynthetic; therefore, the species is photogenic D. If two species are members of the same genus, they are more alike than each of the them could be to a different genus | back 28 C. Hundreds of individuals of a species have been observed and all are photosynthetic; therefore, the species is photogenic |
front 29 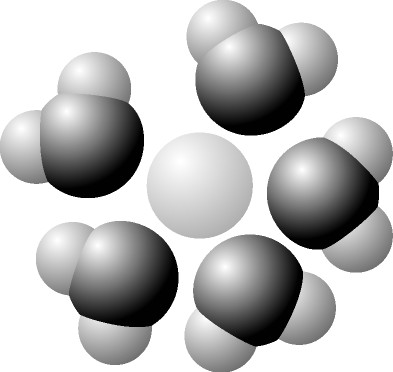 Based on your knowledge of the polarity of water molecules, the
solute molecule depicted here is most likely | back 29 A. Positively Charged |
front 30 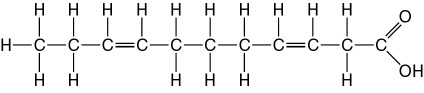 The molecule illustrated in the accompanying figure _____. A. is a saturated fatty acid B. will be liquid at room temperature C. is a carbohydrate D. stores genetic information | back 30 B. will be liquid at room temperature |
front 31 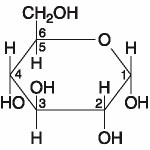 The molecule shown in the accompanying figure is _____. A. Fructose B. Maltose C. a hexose D. a pentose | back 31 C. a hexose |
front 32 If one strand of a DNA molecule has the sequence of bases 5'ATTGCA3',
the other complementary strand would have the sequence | back 32 B. 5'TGCAAT3'. |
front 33 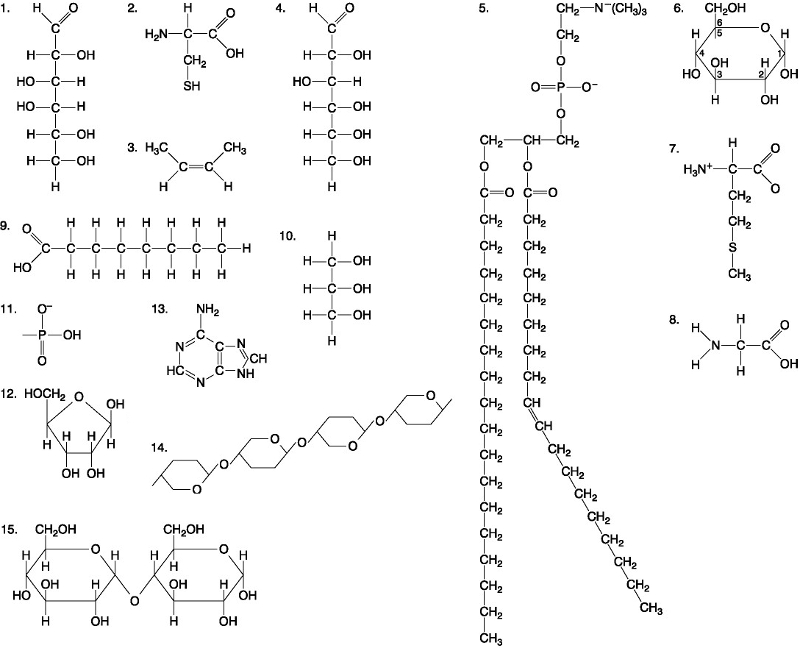 Which molecule is a saturated fatty acid? A. 5 B. 8 C. 9 D. 1 | back 33 C. 9 |
front 34 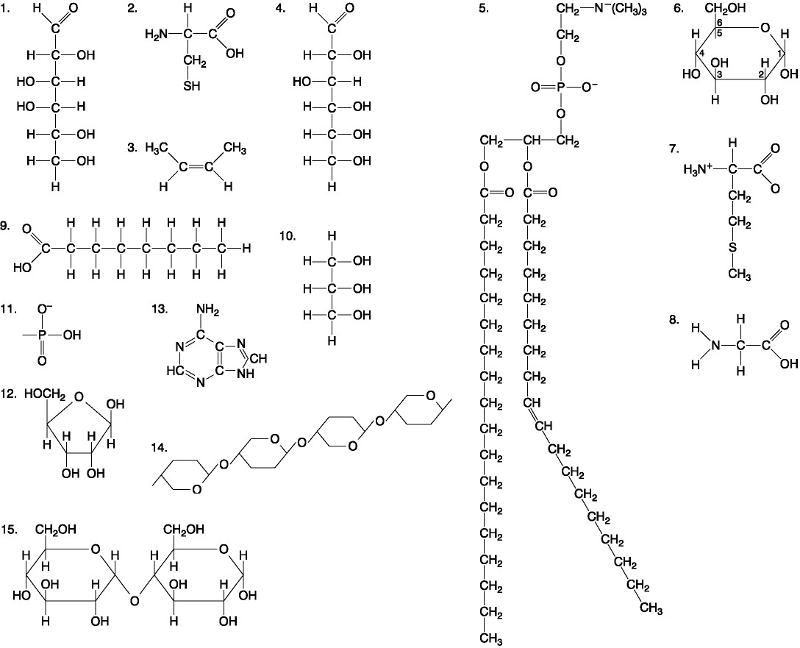 Which molecule has both hydrophilic and hydrophobic properties and is found in plasma? A. 5 B. 12 C. 11 D. 1 | back 34 A. 5 |
front 35 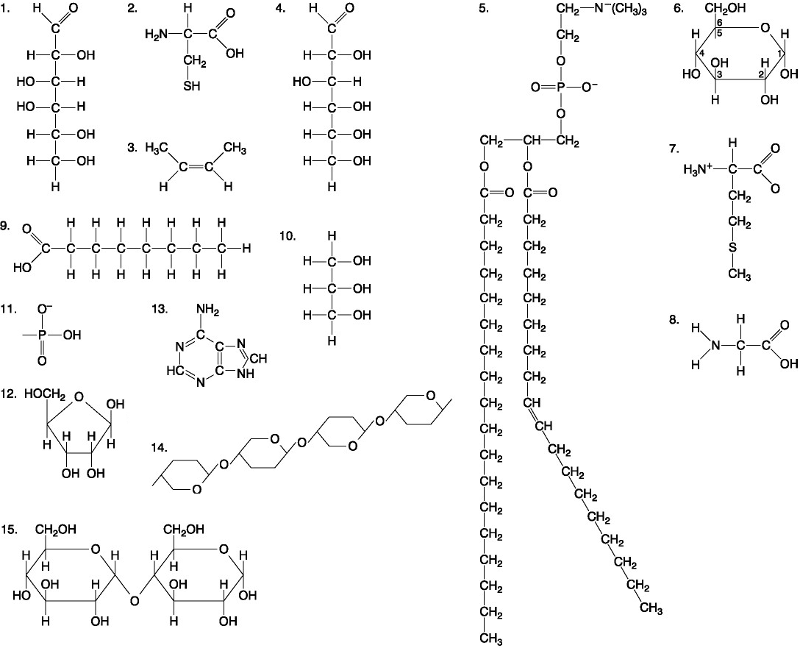 Which of the following combinations of molecules illustrated could be linked to form a nucleotide? A. 3, 7, and 8 B. 5, 9, and 10 C. 11, 12, and 13 D. 1, 2, and 11 | back 35 C. 11, 12, and 13 |
front 36 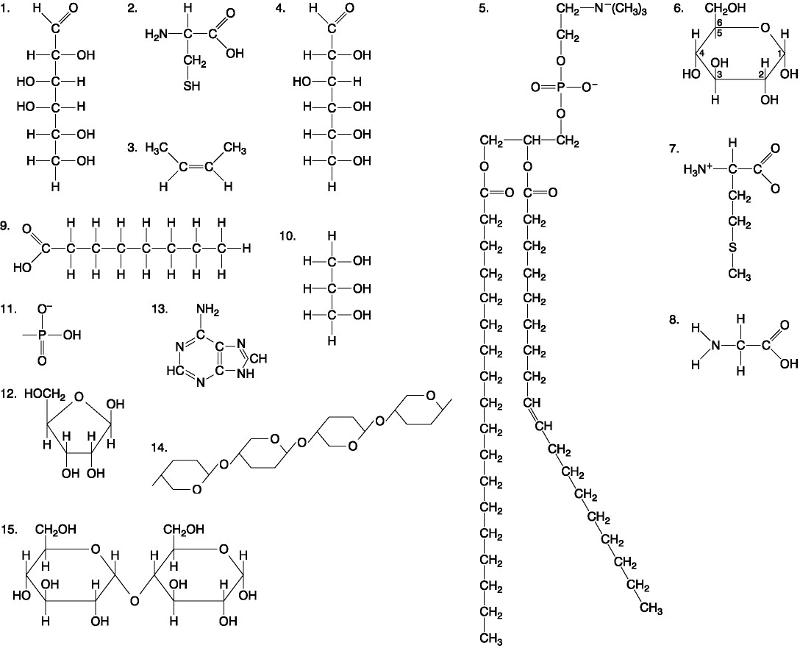 A fat would be formed as a result of a dehydration reaction between _______. A. three molecules of 9 and one molecule of 10 B. one molecule of 5 and three molecules of 9 C. one molecule of 5 and three molecules of 10 D. one molecule of 9 and three molecules of 10 | back 36 A. three molecules of 9 and one molecule of 10 |
front 37 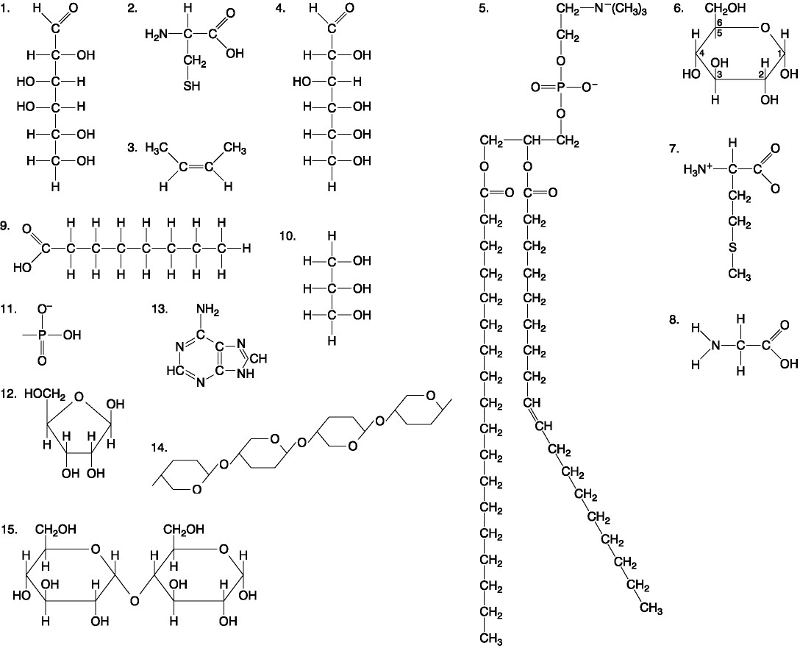 Which of the following molecules is the pentose sugar found in RNA? A. 12 B. 2 C. 13 D. 6 | back 37 C. 13 |
front 38 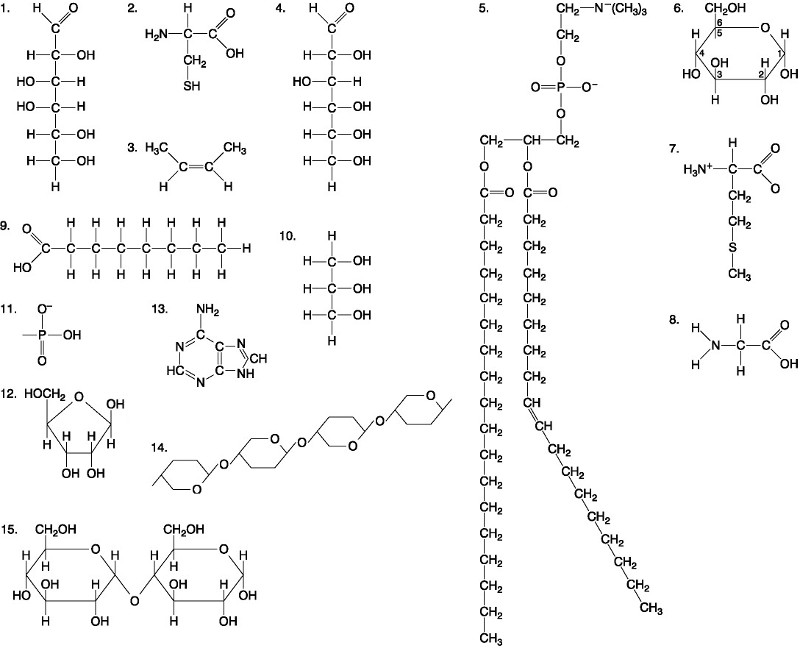 Which of the following molecules act as building blocks of polypeptides? A. 1, 4, and 6 B. 11, 12, and 13 C. 2, 7, and 8 D. 7, 8, and 13 | back 38 C. 2, 7, and 8 |
front 39 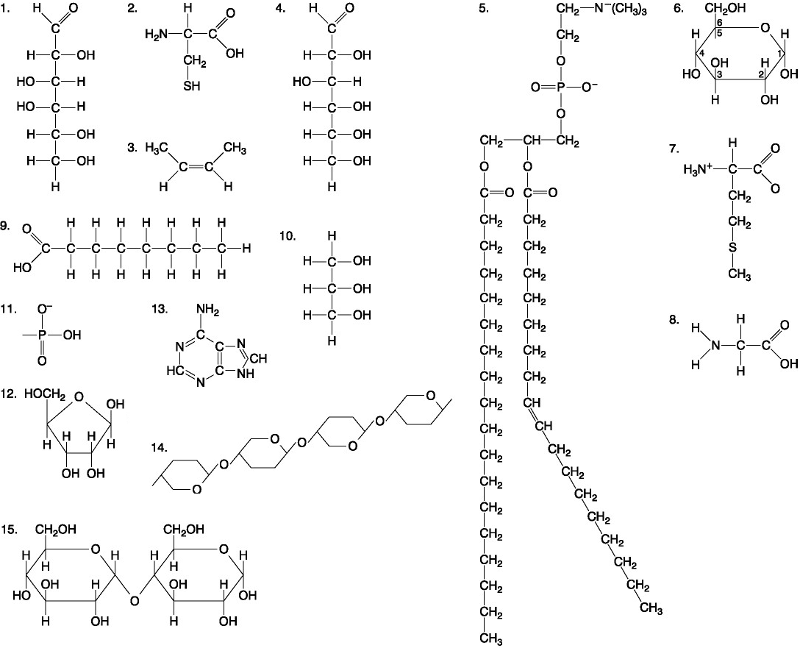 Which of the following pairs of molecules could be joined together by a peptide bond in a dehydration reaction? A. 2 and 2 B. 12 and 13 C. 8 and 9 D. 7 and 8 | back 39 D. 7 and 8 |
front 40 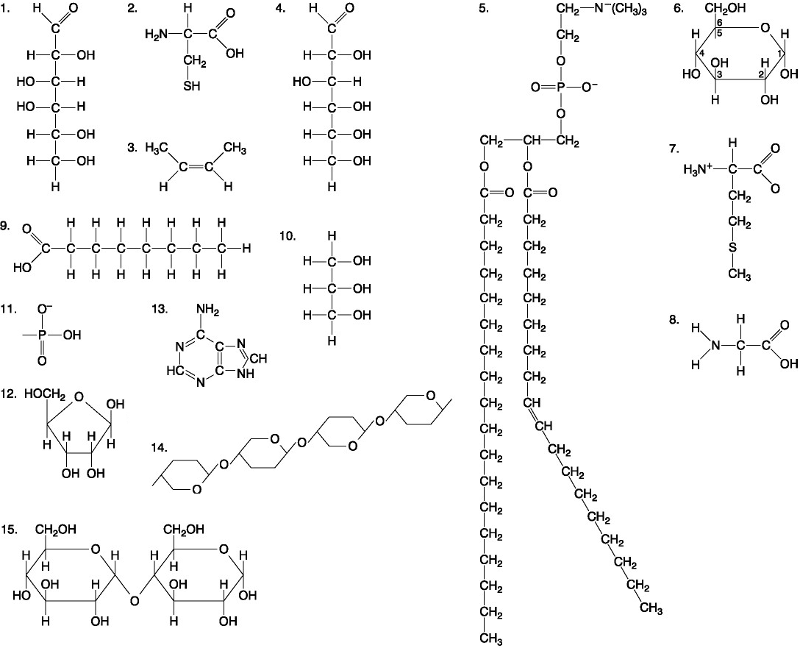 Which one of the following molecules is a purine nitrogenous base? A. 5 B. 13 C. 12 D. 2 | back 40 B. 13 |
front 41 Which one of the following is NOT a component of each monomer used to make proteins? A. a side chain, R B. a phosphorous atom, P C. an amino functional group, NH2 D. a carboxyl group, COOH | back 41 B. a phosphorous atom, P |
front 42 Which of the following descriptions best fits the class of molecules
known as nucleotides? | back 42 E. a nitrogenous base, a phosphate group, and a pentose sugar |
front 43 The molecular formula for glucose is C6H1206. What would be the
molecular formula for a molecule made by linking three glucose
molecules together by dehydration reactions? | back 43 A. C18 H32 016 |
front 44 Which level of protein structure do the "a" helix and the
"B" pleated sheet represent? | back 44 A. Secondary |
front 45 Which of the following is the strongest evidence that protein structure and function are correlated? A. Protiens have four distinct levels of structure and many functions B. Denatured (unfolded) proteins do not function normally C. Enzymes tend to be globular in shape D. Proteins function best at certain temperatures | back 45 B. Denatured (unfolded) proteins do not function normally |
front 46 The R-group, or side chain, of the amino acid serine is -CH2-OH. The
R-group, or side chain, of the amino acid leucine is -CH2-CH-(CH3)2.
Where would you expect to find these A. Leucine would be in the interior, and serine would be on the exterior of the globular protein. B. Serine and leucine would both be on the exterior of the globular protein C. Serine and leucine would bothbe in the interior of the globular protein D. Serine would be in the interior, and leucine would be onteh exterior of the globular protein | back 46 A. Leucine would be in the interior, and serine would be on the exterior of the globular protein. |
front 47 The label on a container of margarine lists "hydrogenated vegetable oil" as the major ingredient. What is the result of adding hydrogens to vegetable oil? A. is less likely to clog artieries B. has fewer trans fatty acids C. has more "kinks" in the fatty acid chains D. is solid at room temperature | back 47 D. is solid at room temperature |
front 48 Which of the following best summarizes the relationship between dehydration reactions and hydrolysis? A. Dehydration reactions and hydrolysis reactions assemble polymers from monomers B. Hydrolysis reactions create polymers and dehydration reactions create monomers C. Dehydration reactions assemble polymers; hydrolysis reactions break polymers apart D. Dehydration reacations eliminate water from membranes; hydrolysis reactions add water to membranes | back 48 C. Dehydration reactions assemble polymers; hydrolysis reactions break polymers apart |
front 49 What component of amino acid structure varies among different amino acids? A. the glycerol molecule that forms the backbone of amino acid B. the long carbon-hydrogen tails of the molecule C. the presence of central C atom D. the components of the R-group | back 49 D. the components of the R-group |
front 50 What is the term used for a protein molecule that assists in the proper folding of other proteins? A. tertiary protein B. renaturing protein C. denaturing protein D. chaperonin | back 50 D. Chaperonin |
front 51 Which of the following statements best summarizes the differences between DNA and RNA? A. DNA contains the base uracil, whereas RNA contains the base thymine B. DNA encodes hereditary information, whereas RNA does not C. The base in DNA contain sugars, whereas the bases in RNA do not contain sugar D. DNA nucleotides contain a different sugar than RNA nucleotides | back 51 D. DNA nucleotides contain a different sugar than RNA nucleotides |
front 52 A new organism is discovered in the forests of Costa Rica. Scientists there determine that the polypeptide sequence of hemoglobin from the new organism has 72 amino acid differences from humans, 65 differences from a gibbon, 49 differences from a rat, and 5 differences from a frog. These data suggest that the new organism A. humans than to frogs B. gibbons than to rats C. rats than to frogs D. frogs than to humans | back 52 D. Frogs than to humans |
front 53 If a DNA sample were composed of 10% thymine, what would be the percentage of guanine? A. 10 B. 80 C. 40 D. it is impossible to answer from the information given | back 53 C. 40 |
front 54 A glycosidic linkage is analogous to which of the following proteins? A. a disulfide bond B. a peptide bond C. A "B"-pleated sheet D. an amino group | back 54 B. a peptide bond |
front 55 Consider two solutions: solution X has a pH of 4; solution Y has a pH of 7. From this information, we can reasonably conclude that _____. A. the concentration of H ions in a solution X is 3 time as great as the concentration of H ions in solution Y B. solution Y has no free H ions C. the concentration of H ions in a solution X is 1000 times as great as the concentration of H ion in solution Y D. the concentration of H ions in solution Y is 1000 times as great as the concentration in H ions in solution X | back 55 C. the concentration of H ions in a solution X is 1000 times as great as the concentration of H ion in solution Y |
front 56 How does a scientific theory differ from a scientific hypothesis? A. Hypotheses are usually an explanation for a more general phenomenon; theories typically address more specific issues B. Theories are proposed to test scientific hypothesis C. Theories are usually an explanation for a more general phenomenon; hypotheses typically address more specific issues D. Confirmed theories become scientific laws; hypothesis become theories | back 56 C.Theories are usually an explanation for a more general phenomenon; hypotheses typically address more specific issues |
front 57 Which of the following effects is produced by the high surface tension of water? A. Organisms can resist temperature changes, although they give off heat due to chemical reactions B. A raft spider can walk across the surface of a small pond C. Lakes cannot freeze solid in winter, despite low temperatures D. Sweat can evaporate from the skin, helping to keep people form overheating | back 57 B. A raft spider can walk across the surface of a small pond |
front 58 In what way are elements in the same column of the periodic table the same? They have the same number of _____. A. electron shells when neutral B. electrons in their valance shells when neutral C. protons D. electrons when neutral | back 58 B. electrons in their valance shells when neutral |