Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Biology Final
front 1 Systems Biology is mainly an attempt to: | back 1 Understand the behavior of entire biological systems |
front 2 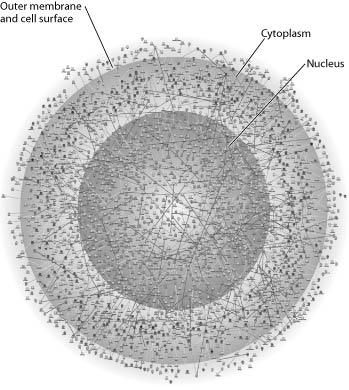 The illustration above most probably represents: | back 2 a map of a network of protein interactions within a eukaryotic cell |
front 3 Organisms interact with their environments, exchanging matter and energy. For example, Plant chloroplasts convert the energy of sunlight into | back 3 The potential energy of chemical bonds |
front 4 The application of scientific knowledge for some specific purpose is known as | back 4 Technology |
front 5 Which of the following categories of organisms is least likely to be revised? | back 5 Species |
front 6 Which of the following best describes a model organism? | back 6 It is well studied, easy to grow, and results are widely applicable. |
front 7 When a hypothesis cannot be written in an "if...then" format, what does this mean? | back 7 It does not represent deductive reasoning |
front 8 Through time, the lineage that led to modern whales shows a change from four- limbed land animals to aquatic animals with two limbs that function as flippers. this change is best explained by: | back 8 Natural Selection |
front 9 Which of these is an example of inductive reasoning? | back 9 Hundreds of individuals of a species have been observed and are all photosynthetic; therefore, the species is photosynthetic. |
front 10  In a high school laboratory. which of the following constitutes an experiment? | back 10 III only |
front 11 We can represent atoms by listing the number of protons, neutrons, and electrons for example, 2p+; 2n0; 2e- for helium. Which of the following represents the 18O isotope of oxygen? | back 11 8p+, 10n0, 8e- |
front 12 If a salamander relied on hydrogen bonds to cling to surfaces, what type of surface would cause the most problems for this animal? | back 12 The surface of hydrocarbons |
front 13 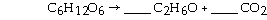 What coefficients must be placed in the following blanks so that all atoms are accounted for in the products? | back 13 2,2 |
front 14 which of these systems is least likely to be at chemical equilibrium? | back 14 a test tube of living cells |
front 15 How many electron pairs are shared between carbon atoms in a molecule that has the formula C2H4? | back 15 2 |
front 16 About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living matter? | back 16 Carbon, hydrogen, nitrogen, oxygen |
front 17 Which of the following molecules contains the most polar covalent bond? | back 17 H2O |
front 18 In comparing covalent bonds and ionic bonds, which of the following would you expect? | back 18 Covalent bonds and ionic bonds occupy opposite ends of a continuous spectrum, from nearly equal to completely unequal sharing of electrons |
front 19 What is the difference between covalent bonds and ionic bonds? | back 19 Covalent bonds involve the sharing of electrons between atoms, ionic bonds involve the electrical attraction between atoms. |
front 20 Trace elements are those required by an organism in only minute quantities. Which of the following is a trace element that is required by humans and other vertebrates, but not by other organisms such as bacteria or plants? | back 20 Iodine |
front 21  what does this picture and the mother of pearl (the plant of front of your textbook) have in common? | back 21 adaptions to conserve water |
front 22 A localized group of organisms that belong to the same species is called a | back 22 population |
front 23 Protists and bacteria are grouped into different domains because | back 23 protists have a membrane-bounded nucleus, which bacterial cells lack. |
front 24 A water sample from a hot thermal vent contained a single-celled organism that had a cell wall but lacked a nucleus. What is its most likely classification? | back 24 Archaea |
front 25 Which of the following is an example of qualitative data? | back 25 The fish swam in a zigzag motion |
front 26 Why is a scientific topic best discussed by people of varying points of view, a variety of subdisciplines, and diverse cultures? | back 26 Robust and critical discussion between diverse groups improves scientific thinking |
front 27 In a hypothetical world, every 50 years people over 6 feet tall are eliminated from the population before they reproduce. Based on your knowledge of natural selection, you would predict that the average height of the human population will | back 27 gradually decline |
front 28 Charles Darwin proposed a mechanism for descent with modification that stated that organisms of a particular species are adapted to their environment when they possess | back 28 inheritable traits that enhance their survival and reproductive success in the local environment |
front 29 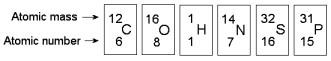 Based on electron configuration, which of these elements in the figure above would exhibit a chemical behavior most like that of oxygen? | back 29 Sulfur |
front 30 A covalent chemical bond is one in which | back 30 outer-shell electrons of two atoms are shared so as to satisfactorily fill the outer electron shells of both atoms. |
front 31 Atoms whose outer electron shells contain 8 electrons tend to | back 31 be both chemically inert and gaseous at room temperature. |
front 32 Electrons exist only at fixed levels of potential energy. However, if an atom absorbs sufficient energy, a possible result is that | back 32 an electron may move to an electron shell farther away from the nucleus |
front 33 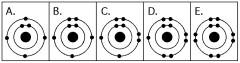 Which drawing in the figure above depicts the electron configuration of an element with chemical properties most similar to Helium (2He)? | back 33 E |
front 34 The atomic number of sulfur is 16. Sulfur combines with hydrogen by covalent bonding to form a compound, hydrogen sulfide. Based on the number of valence electrons in a sulfur atom, predict the molecular formula of the compound: | back 34 H2S |
front 35 What is the maximum number of hydrogen atoms that can be covalently bonded in a molecule containing two carbon atoms? | back 35 6 |
front 36 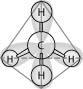 In the methane molecule shown in the figure above, bonds have formed that include both the s orbital valence electrons of the hydrogen atoms and the p orbital valence electrons of the carbon. The electron orbitals in these bonds are said to be | back 36 Hybrid orbitals |
front 37 The organic molecules in living organisms have a measurably lower ratio of carbon-13/carbon-12, two stable isotopes of carbon that comprise approximately 1.1% and 98.9% of atmospheric carbon, respectively. What is a reasonable explanation for this phenomenon? | back 37 Photosynthesis preferentially uses carbon dioxide molecules with carbon-12, and the lower carbon-13/carbon-12 ratio propagates through the food chain |
front 38 An atom has 6 electrons in its outer shell. How many unpaired electrons does it have? | back 38 2 |
front 39 Knowing just the atomic mass of an element allows inferences about which of the following? | back 39 The number of protons plus neutrons in an element |
front 40 Oxygen has an atomic number of 8 and a mass number of 16. Thus, what is the atomic mass of an oxygen atom? | back 40 approximately 16 Daltons |
front 41 Prokaryotic and eukaryotic cells generally have which of the following features in common? | back 41 ribosomes |
front 42 A controlled experiment is one in which | back 42 there are at least two groups, one of which does not receive the experimental treatment. |
front 43 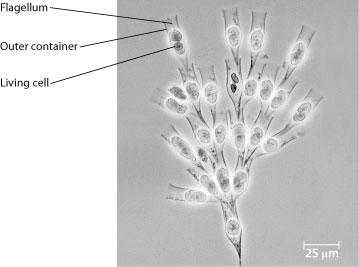 Use the following information to answer the questions below. Golden algae are a group of protists whose color is due to carotenoid pigments: yellow and brown. Most have two flagella and all are photosynthetic. A group of students was given a significant sample of one of these (Dinobryon) that is colonial. Their instructions for the project were to design two or more experiments that could be done with these organisms. The students decide that for one of their experiments, they want to see whether the organisms can photosynthesize. Which of the following is the best hypothesis? | back 43 The organisms are photosynthesis |
front 44 Given the cooperativity of science, which of the following is most likely to result in an investigator being intellectually looked down upon by other scientists? | back 44 Being found to have falsified or created data to better fit a hypothesis. |
front 45  The following is a list of biology themes discussed in Chapter 1. Use them to answer the following questions. | back 45 I and II |
front 46 What are archaea? | back 46 prokaryotes characterized as extremophiles that share some bacterial and some eukaryotic traits |
front 47 Once labor begins in childbirth, contractions increase in intensity and frequency until delivery. The increasing labor contractions of childbirth are an example of which type of regulation? | back 47 positive feedback |
front 48 The atomic number of chlorine is 17. The atomic number of magnesium is 12. What is the formula for magnesium chloride? | back 48 MgCl2 |
front 49 A covalent bond is likely to be polar when | back 49 one of the atoms sharing electrons is much more electronegative than the other atom |
front 50 Which statement is true of all atoms that are anions? | back 50 The atom has more electrons than protons |
front 51  Which of the following best describes the relationship between the atoms described below? | back 51 They are isotopes |
front 52 All the organisms on your campus make up | back 52 a community |
front 53 The main source of energy for producers in an ecosystem is: | back 53 light energy |
front 54 4) Which of the following types of cells utilize DNA as their genetic material but do not have their DNA encased within a nuclear envelope: | back 54 archaea |
front 55 To understand the chemical basis of inheritance, we must understand the molecular stand of DNA. This is an example of the application of which concept to the study of biology? | back 55 reductionism |
front 56 When the body’s blood glucose level rises, the pancreas secretes insulin and, as a result, the blood glucose level declines. When the blood glucose level is low, the pancreas secrets glucagon and the blood glucose level rises. Such regulation of the blood glucose level is the result of: | back 56 negative feedback |
front 57 Which branch of biology is concerned with the naming and classifying of organisms? | back 57 Taxonomy |
front 58 Prokaryotes are classified as belonging to two different domains. What are the domains? | back 58 Bacteria and Archaea |
front 59 Global warning, as demonstrated by observation such as melting of glaciers, increasing CO2 levels, and increasing average ambient temperatures, has already had many effects on living organisms. Which of the following might best offer a solution to this problem? | back 59 Limit the burning of fossil fuels and regulate the loss of our forested areas |
front 60 A filamentous organism has been isolated from decomposing organic matter. The organism has a cell but no chloroplast. How would you classify this organism? | back 60 Domain Eukarya, kingdom fungi |
front 61 Which of these provides evidence of the common ancestry of all life? | back 61 Near universality of genetic code |
front 62 Which of the following is true about natural selection: | back 62 It requires genetic variation, results in decent with modification, and involves differential reproductive success |
front 63 Which of these individuals is likely to be most successful in an evolutionary sense? | back 63 An organism that dies after five days of life but leaves 10 offspring, all of whom survive to reproduce |
front 64 What is the major difference between a kingdom and a domain? | back 64 All eukarya belong to one domain |
front 65 One difference between carbon -12 (12/6 C) is that carbon -14 (14/6 C) has | back 65 two or more neutrons |
front 66 A group of molecular biologists is trying to synthesize a new artificial compound to mimic the effects of a known hormone that influences sexual ___ for advice. Which of the following compounds is most likely to mimic the effects of a hormone? | back 66 A compound with the same three- dimensional shape as part of the hormone |
front 67 Carbon- 12 is the most common isotope of carbon, and has an atomic mass of 12 Daltons a mole of carbon in natural occurring carbon however weighs slightly more than 12 grams. Why? | back 67 Some carbon atoms in nature have a different valence electron distribution |