Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Biology Exam ll (Chapter 6)
front 1 Bioenergetics | back 1 Energy in biological systems |
front 2 Flow of Energy | back 2 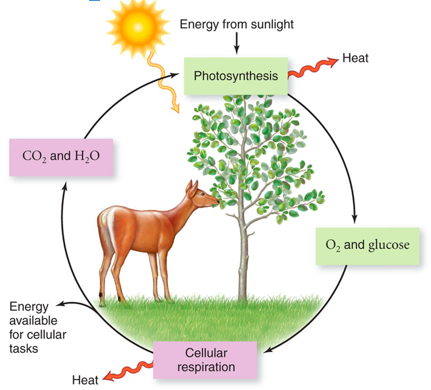 |
front 3 What is energy in the form of? | back 3 Energy is in the form of a photon. |
front 4 Energy | back 4 The ability to do work which means to move matter against opposing forces such as gravity and friction |
front 5 Carbon Cycle | back 5 Transforms carbon dioxide into glucose |
front 6 First Law of Thermodynamics | back 6 Energy cannot be created or destroyed |
front 7 Potential Energy | back 7 ATP, an electrical/ion gradient, concentration gradient, NADH, Chemical Bonds
|
front 8 Pi + ADP ==> ATP | back 8 It has a change in free energy that is greater than 0. That means it has a delta G that is positive and therefore the reaction is endergonic. |
front 9 Kinetic Energy | back 9 Movement
|
front 10 Noncompetitive Inhibitor | back 10 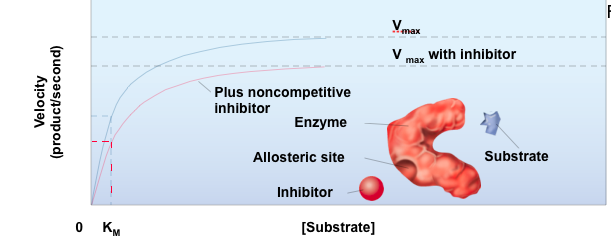 It lowers the Vmax and has no affect on the Km.
|
front 11 Altering the three-dimensional structure of an enzyme might | back 11 Prevent the substrate from binding the enzyme's active site |
front 12 Enzymes | back 12 Increase the rate of the reaction by reducing the activation energy.
|
front 13 What are enzymes made of? | back 13 Mostly proteins but some are RNA molecules possess enzymatic functions called ribozymes |
front 14 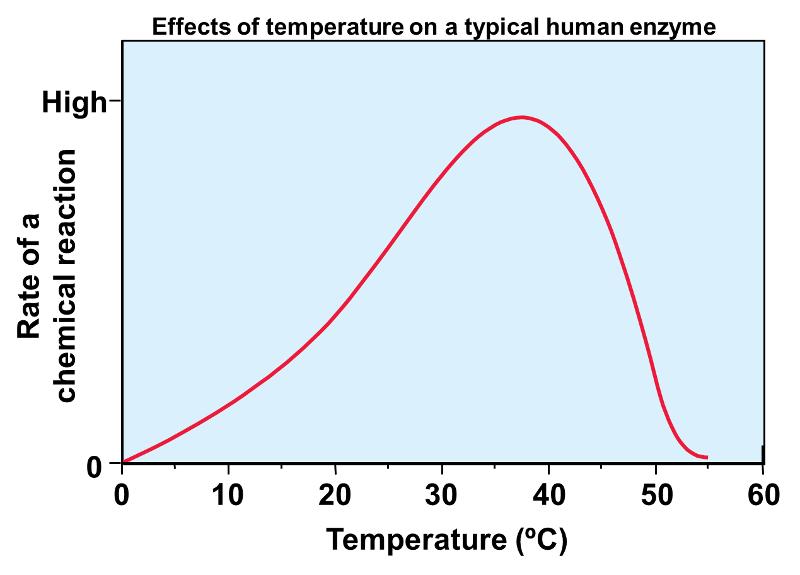 | back 14 This enzyme's optimal function is at about 37 degrees C, the enzymatic activity of the enzymes slows down around 40 C |
front 15 NAD+ + H+ --> NADH
| back 15 It has been reduced |
front 16 Free energy
| back 16 The amount of available energy that can be used to promote change do work |
front 17 How do you overcome the effect of a competitive inhibitor on enzyme activity | back 17 Increase the amount of substrate (Km) for the enzyme |
front 18 Positive ∆G | back 18 Favors formation of reactants |
front 19 Negative ∆G | back 19 Favors formation of products |
front 20 Steps of an enzyme-catalyzed reaction | back 20 1. substrates bind to enzyme; 2. enzyme and substrate reach transtition state; 3. substrates are converted to products; 4. products are released |
front 21 Active Site of Enzyme | back 21 Where the chemical reaction takes place
|
front 22 Second Law of Thermodynamics | back 22 Every chemical reaction must increase the total entropy of the universe. Every chemical reaction represents a transfer of energy, which increases entropy
|
front 23 Competitive Inhibitor | back 23 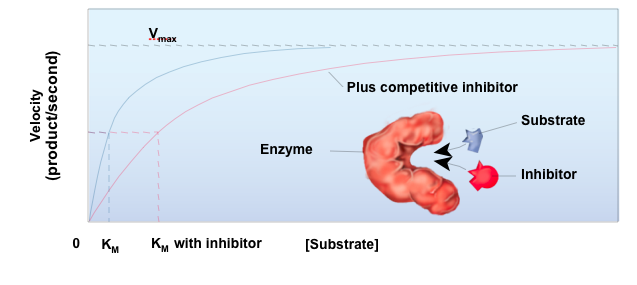 Only raises the Km
|
front 24 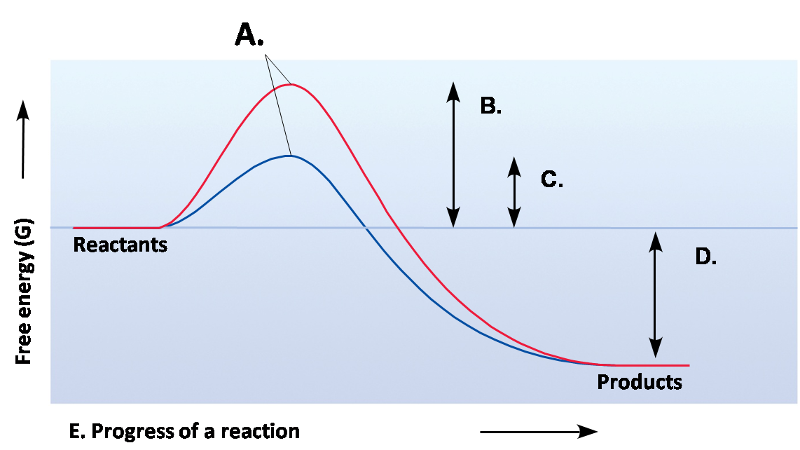 | back 24 Exergonic |
front 25 NADH is converted to NAD+ and H+. What has happened to NADH? | back 25 It has been oxidized. |
front 26 Entropy | back 26 Measure of disorder |
front 27 Exergonic Reaction | back 27 ∆G is negative/less than zero
|
front 28 Endergonic Reaction | back 28 ∆G is positive/greater than zero
|
front 29 ATP (Adenosine Triphosphate) | back 29 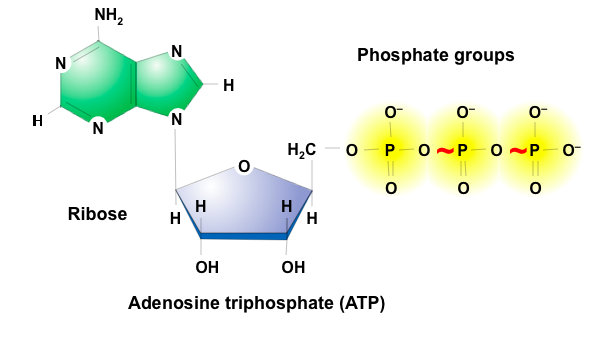 Universal energy molecule
|
front 30 Change in free energy determines what? | back 30 The direction of chemical reactions |
front 31 ∆G = | back 31 ∆H - T∆S |
front 32 ∆G | back 32 Free energy |
front 33 ∆H | back 33 Total energy |
front 34 T | back 34 Absolute temperature |
front 35 ∆S | back 35 Entropy |
front 36 Energy emitted through _______ is not usable. | back 36 Heat |
front 37 Energy Systems | back 37 Are not efficient |
front 38 Hydrolysis of ATP requires what to be exergonic? | back 38 Water and enzymes
|
front 39 Glucose + Phosphate --> glucose-phosphate + H2O | back 39 ΔG = +3.3 Kcal/mole and it is an endergonic reaction |
front 40 Glucose + ATP → glucose-phosphate + ADP | back 40 Coupled Reaction |
front 41 Phosphorylation | back 41 Direct transfer of a phosphate group from ATP to a substrate (an example being glucose)
|
front 42 Spontaneous Reaction | back 42 Does not imply anything about its speed
|
front 43 Catalyst | back 43 Speed up rate of reaction without being consumed |
front 44 Names of enzymes usually end in ________ and typically describe what is going on. | back 44 -ASE |
front 45 Activation Energy | back 45 Initial input of energy to start a reaction
|
front 46 Transition State | back 46 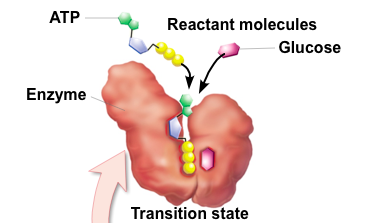 Bonds are stretched/strained |
front 47 2 ways to overcome activation energy: | back 47 1. Large amounts of heat
|
front 48 How to measure enzyme activity: | back 48 Measure your substrates and your products |
front 49 VMax | back 49 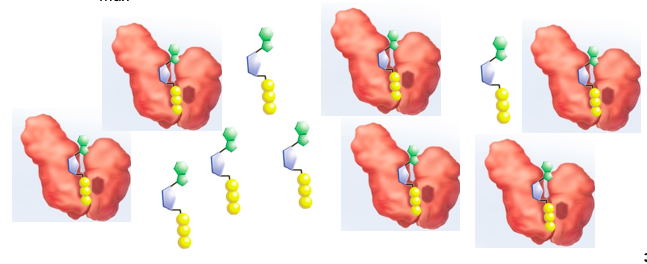 Velocity of reaction near maximum rate |
front 50 Km | back 50  Substrate concentration at which VMax is at half of max rate
|
front 51 If Km is high, the enzyme needs ______. This means the enzyme has a low affinity for the substrate. | back 51 A lot of substrate |
front 52 Saturation | back 52 Plateau where nearly all active sites are occupied by substrates |
front 53 Why do cells use inhibitors? | back 53 To turn off or slow down an enzyme |
front 54 Coenzyme | back 54 Enzyme "helper"
|
front 55 Cofactor | back 55 Enzyme "helper"
|
front 56 Enzyme helpers (coenzymes and cofactors) will _______. | back 56 Bind to the enzyme or participate in the reaction |
front 57 Vitamin C | back 57 Functions in muscle formation (collagen)
|
front 58 Vitamin B3 | back 58 "Nicotine acid"
|
front 59 Which structures do enzymes most heavily depend on? | back 59 Tertiary and quaternary |
front 60 Denature | back 60 When structure is lost due to heat
|
front 61 The shape of the enzyme is altered by _____? | back 61 1. pH - Measure of H+ (0-7 is acidic and 8-14 is basic)
|
front 62 Metabolism | back 62 Each step is coordinated by a specific enzyme |
front 63 2 types of metabolism | back 63 1. Catabolic Pathways (Reactions)
|
front 64 Catabolic Pathways (Reactions) | back 64 Breaks down reactants
|
front 65 Anabolic Pathways (Reactions) | back 65 Promote synthesis (builds up, not breaks down)
|
front 66 Protease | back 66 Turns proteins into amino acids (catabolic pathway) |
front 67 Nuclease | back 67 Breaks down RNA into nucleotides (catabolic pathway) |
front 68 Hydrolysis of ATP ______ energy. | back 68 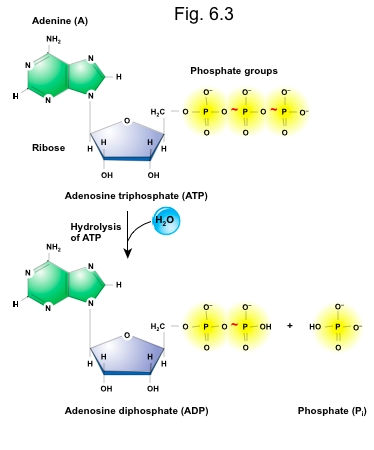 Releases
|
front 69 Synthesis of ATP _______ energy. | back 69 Requires
|
front 70 Phosphorylation (substrate level) | back 70 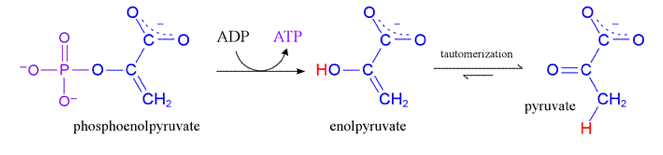 Finds a free floating Pi and takes it, which converts ADP to ATP |
front 71 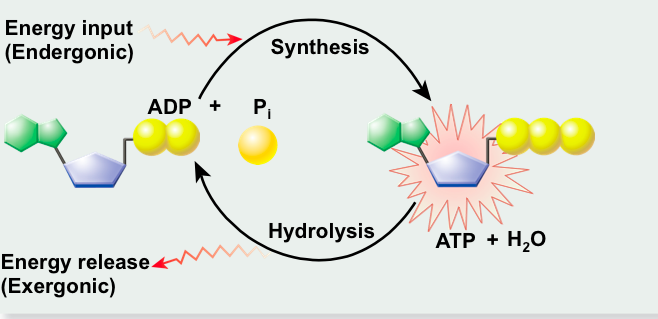 | back 71 ATP Synthesis |
front 72 Chemiosmosis | back 72 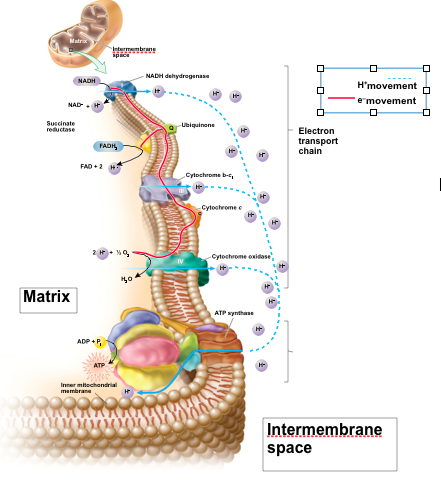 Deals with an electrochemical gradient |
front 73 Intermediates | back 73 Compounds formed between initial reactants & products |
front 74 Thermodynamics | back 74 Study of energy transformation |
front 75 Enzyme-Substrate Complex | back 75 When an enzyme and a substrate come together
|
front 76 Enzyme action time is proportional to the concentration of the substrate: | back 76 The more substrate you have, the faster the reaction rate will be (until you reach saturation) |
front 77 Enzyme Inhibitors | back 77 Chemicals that interfere with enzyme function
|
front 78 Allosteric site | back 78 Site on the enzyme that isn't the active site
|
front 79 Energy Intermediates: Redox
| back 79 Electron is removed from one molecule and added to another molecule |
front 80 Oxidation (oil) | back 80 Removal of electrons |
front 81 Reduction (Rig) | back 81 Addition of electrons |
front 82 Ae- + B → A + Be-
| back 82 Oxidized (electron removed) |
front 83 Ae- + B → A + Be-
| back 83 Reduced (electron added) |
front 84 Energy Intermediates: NAD | back 84 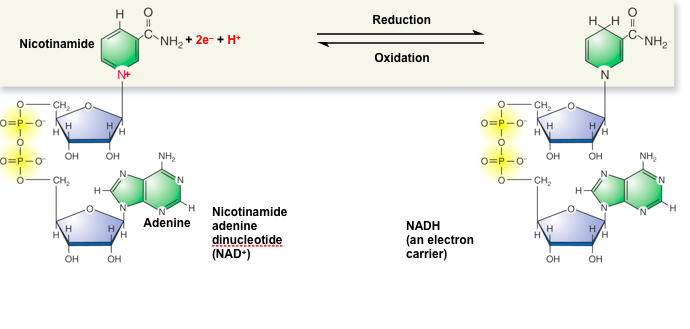 NAD is a type of nucleic acid
|
front 85 Electrons are synonymous with ______ in chemical reactions. | back 85 Energy |
front 86 Where is energy found in molecules? | back 86 Bonds |
front 87 Regulation of metabolic pathways | back 87 3 types:
|
front 88 Gene Regulation | back 88 Turn genes on or off |
front 89 Cellular Regulation | back 89 Cell-signaling pathways like hormones |
front 90 Biochemical Regulation | back 90 Feedback Inhibition- Product of pathway inhibits early steps to prevent over accumulation of product |
front 91 Concentration Gradient | back 91 Refers to a differences of solutes (dissolved substances) in an adjacent area |
front 92 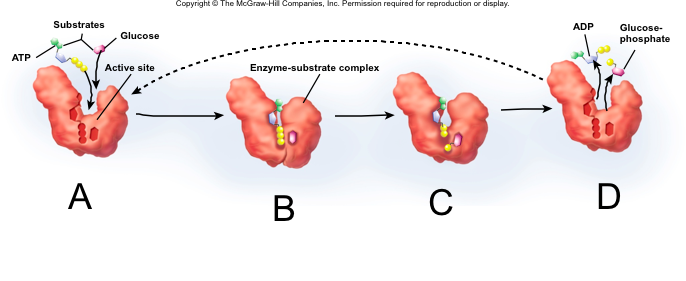 Enzyme-Catalyzed Reaction | back 92 A. Substrate binding
|