Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Final Bio Testing Unit
front 1 group of molecular biologists is trying to synthesize a new artificial compound to mimic the effects of a known hormone that influences sexual behavior. They have turned to you for advice. Which of the following compounds is most likely to mimic the effects of the hormone?
| back 1 Answer: C |
front 2 The complexity and variety of organic molecules is due to
| back 2 Answer: A |
front 3 Differences among organisms are caused by
| back 3 Answer: B |
front 4 Which of the following statements correctly describes cis-trans isomers?
| back 4 Answer: A |
front 5 Research indicates that ibuprofen, a drug used to relieve inflammation and pain, is a mixture of two enantiomers; that is, molecules that
| back 5 Answer: B |
front 6 What determines whether a carbon atom's covalent bonds to other atoms are in a tetrahedral configuration or a planar configuration?
| back 6 Answer: B |
front 7 Compared to a hydrocarbon chain where all the carbon atoms are linked by single bonds, a hydrocarbon chain with the same number of carbon atoms, but with one or more double bonds, will
| back 7 Answer: B |
front 8 Organic molecules with only hydrogens and five carbon atoms can have different structures in all of the following ways except
| back 8 Answer: E |
front 9 A compound contains hydroxyl groups as its predominant functional group. Which of the following statements is true concerning this compound?
| back 9 Answer: B |
front 10 Which of the following is a false statement concerning amino groups?
| back 10 Answer: D |
front 11 Which two functional groups are always found in amino acids?
| back 11 Answer: C |
front 12 Amino acids are acids because they always possess which functional group?
| back 12 Answer: C |
front 13 Which functional groups can act as acids?
| back 13 Answer: C |
front 14 Testosterone and estradiol are
| back 14 Answer: B |
front 15 Testosterone and estradiol are male and female sex hormones, respectively, in many vertebrates. In what way(s) do these molecules differ from each other?
| back 15 Answer: C |
front 16 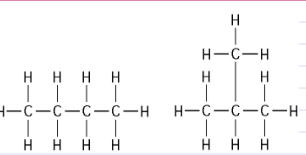 The two molecules shown in the figure above are best described as
| back 16 Answer: C |
front 17 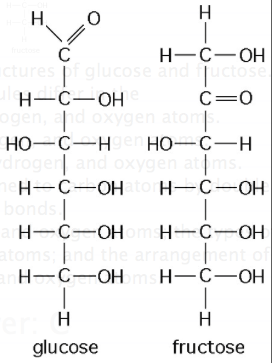 he figure above shows the structures of glucose and fructose. These two molecules differ in the
| back 17 Answer: C
|
front 18 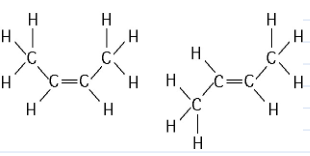 The two molecules shown in the figure above are best described as
| back 18 Answer: E |
front 19 Thalidomide and L-dopa, shown below, are examples of pharmaceutical drugs that occur as enantiomers, or molecules that
| back 19 Answer: B |
front 20 O=C-O-H
| back 20 Carboxyl |
front 21 A. -OH B. C=O C. O=C-O-H D. -NH2 E. -SH
| back 21 Answer: A |
front 22 A. -OH B. C=O C. O=C-O-H D. -NH2 E. -SH
| back 22 Answer: E |
front 23 A. -OH B. C=O C. O=C-O-H D. -NH2 E. -SH
| back 23 Answer: B |
front 24 A. -OH B. C=O C. O=C-O-H D. -NH2 E. -SH
| back 24 Answer: E |
front 25 A. -OH B. C=O C. O=C-O-H D. -NH2 E. -SH
| back 25 Answer: C |
front 26 A. -OH B. C=O C. O=C-O-H D. -NH2 E. -SH
| back 26 Answer: C |
front 27 A. -OH B. C=O C. O=C-O-H D. -NH2 E. -SH
| back 27 Answer: D |
front 28 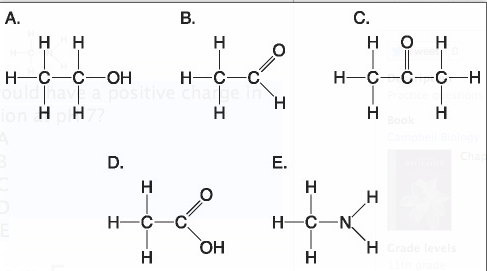 Which molecule shown above would have a positive charge in aqueous solution at pH 7?
| back 28 Answer: E |
front 29 ***Which molecule(s) shown above is (are) ionized in aqueous solution at pH 7?
| back 29 Answer: A |
front 30 ***Which molecules shown above contain a carbonyl group?
| back 30 Answer: B |
front 31 ***Which molecule shown above has a carbonyl functional group in the form of a ketone?
| back 31 Answer: C |
front 32 ***Which molecule shown above has a carbonyl functional group in the form of an aldehyde?
| back 32 Answer:B |
front 33 ***Which molecule shown above contains a carboxyl group?
| back 33 Answer: D |
front 34 Which molecule shown above can increase the concentration of hydrogen ions in a solution and is therefore an organic acid?
| back 34 Answer: D |
front 35 Humans and mice differ because
| back 35 Answer: D |
front 36 Molecules with which functional groups may form polymers via dehydration reactions?
| back 36 Answer: E |
front 37 Which of these molecules is not formed by dehydration reactions?
| back 37 Answer: A |
front 38 ) In animal metabolism, most of the monomers released by digestion of food macromolecules are metabolized to provide energy. Only a small portion of these monomers are used for synthesis of new macromolecules. The net result is that
| back 38 Answer: B |
front 39 What is the chemical reaction mechanism by which cells make polymers from monomers?
| back 39 Answer: C |
front 40 Which of the following best summarizes the relationship between dehydration reactions and hydrolysis?
| back 40 Answer: A |
front 41 All of the following contain amino acids except
| back 41 Answer: B |
front 42 ) The bonding of two amino acid molecules to form a larger molecule requires
| back 42 Answer: A |
front 43 There are 20 different amino acids. What makes one amino acid different from another?
| back 43 Answer: C |
front 44 Dehydration reactions are used in forming which of the following compounds?
| back 44 Answer: E |
front 45 What aspects of protein structure are stabilized or assisted by hydrogen bonds?
| back 45 Answer: E |
front 46 Which bonds are created during the formation of the primary structure of a protein?
| back 46 Answer: A |
front 47 What maintains the secondary structure of a protein?
| back 47 Answer: B |
front 48 What type of covalent bond between amino acid side chains (R groups) functions in maintaining a polypeptide's specific three-dimensional shape?
| back 48 Answer: D |
front 49 Misfolding of polypeptides is a serious problem in cells. Which of the following diseases are associated with an accumulation of misfolded polypeptides?
| back 49 Answer:D |
front 50 Changing a single amino acid in a protein consisting of 325 amino acids would
| back 50 Answer: E |
front 51 Normal hemoglobin is a tetramer, consisting of two molecules of β hemoglobin and two molecules of α hemoglobin. In sickle-cell disease, as a result of a single amino acid change, the mutant hemoglobin tetramers associate with each other and assemble into large fibers. Based on this information alone, we can conclude that sickle-cell hemoglobin exhibits
| back 51 Answer: E |
front 52 Which of the following statements about the 5' end of a polynucleotide strand of DNA is correct?
| back 52 Answer: B |
front 53 One of the primary functions of RNA molecules is to
| back 53 Answer: B |
front 54 If ¹⁴C-labeled uridine triphosphate is added to the growth medium of cells, what macromolecules will be labeled?
| back 54 Answer: C |
front 55 Which of the following descriptions best fits the class of molecules known as nucleotides?
| back 55 Answer: C |
front 56 Which of the following are nitrogenous bases of the pyrimidine type?
| back 56 Answer: B |
front 57 Which of the following are nitrogenous bases of the purine type?
| back 57 Answer: B |
front 58 The difference between the sugar in DNA and the sugar in RNA is that the sugar in DNA
| back 58 Answer: E |
front 59 Which of the following statements best summarizes the differences between DNA and RNA?
| back 59 Answer: C |
front 60 If one strand of a DNA molecule has the sequence of bases 5'ATTGCA3', the other complementary strand would have the sequence
| back 60 Answer: B |
front 61 What is the structural feature that allows DNA to replicate?
| back 61 Answer: B |
front 62 Which of the following is an example of hydrolysis?
| back 62 Answer: D |
front 63 If cells are grown in a medium containing radioactive ³²P-labeled phosphate, which of these molecules will be labeled?
| back 63 Answer: E |
front 64 If cells are grown in a medium containing radioactive ¹⁵N, which of these molecules will be labeled?
| back 64 Answer: E |
front 65 Approximately 32 different monomeric carbohydrate subunits are found in various natural polysaccharides. Proteins are composed of 20 different amino acids. DNA and RNA are each synthesized from four nucleotides.
| back 65 Answer: C |
front 66 Which class of biological polymer has the greatest functional variety?
| back 66 Answer: B |
front 67 Which organelle or structure is absent in plant cells?
| back 67 Answer: D |
front 68 Large numbers of ribosomes are present in cells that specialize in producing which of the following molecules?
| back 68 Answer: C |
front 69 The nuclear lamina is an array of filaments on the inner side of the nuclear membrane. If a method were found that could cause the lamina to fall into disarray, what would you expect to be the most likely consequence?
| back 69 Answer: C |
front 70 A cell with a predominance of free ribosomes is most likely
| back 70 Answer: B |
front 71 Which type of organelle or structure is primarily involved in the synthesis of oils, phospholipids, and steroids?
| back 71 Answer: C |
front 72 Which structure is the site of the synthesis of proteins that may be exported from the cell?
| back 72 Answer: A |
front 73 The Golgi apparatus has a polarity or sidedness to its structure and function. Which of the following statements correctly describes this polarity?
| back 73 Answer: E |
front 74 The fact that the outer membrane of the nuclear envelope has bound ribosomes allows one to most reliably conclude that
| back 74 Answer: A |
front 75 The difference in lipid and protein composition between the membranes of the endomembrane system is largely determined by
| back 75 Answer: C |
front 76 Hydrolytic enzymes must be segregated and packaged to prevent general destruction of cellular components. Which of the following organelles contains these hydrolytic enzymes in animal cells?
| back 76 Answer: B |
front 77 Which of the following statements correctly describes some aspect of protein secretion from prokaryotic cells?
| back 77 Answer: C |
front 78 Tay-Sachs disease is a human genetic abnormality that results in cells accumulating and becoming clogged with very large and complex lipids. Which cellular organelle must be involved in this condition?
| back 78 Answer: C |
front 79 The liver is involved in detoxification of many poisons and drugs. Which of the following structures is primarily involved in this process and therefore abundant in liver cells?
| back 79 Answer: B |
front 80 Which of the following produces and modifies polysaccharides that will be secreted?
| back 80 Answer: D |
front 81 Which of the following contains hydrolytic enzymes?
| back 81 Answer: A |
front 82 Which organelle often takes up much of the volume of a plant cell?
| back 82 Answer: B |
front 83 Which organelle is the primary site of ATP synthesis in eukaryotic cells?
| back 83 Answer: C |
front 84 Which plant cell organelle contains its own DNA and ribosomes?
| back 84 Answer: C |
front 85 The chemical reactions involved in respiration are virtually identical between prokaryotic and eukaryotic cells. In eukaryotic cells, ATP is synthesized primarily on the inner membrane of the mitochondria. In light of the endosymbiont theory for the evolutionary origin of mitochondria, where is most ATP synthesis likely to occur in prokaryotic cells?
| back 85 Answer: D |
front 86 In a liver cell detoxifying alcohol and some other poisons, the enzymes of the peroxisome remove hydrogen from these molecules and
| back 86 Answer: D |
front 87 Motor proteins provide for molecular motion in cells by interacting with what types of cellular structures?
| back 87 Answer: D |
front 88 Centrioles, cilia, flagella, and basal bodies have remarkably similar structural elements and arrangements. Which of the following hypotheses is most plausible in light of such structural similarities?
| back 88 Answer: A |
front 89 Cytochalasin D is a drug that prevents actin polymerization. A cell treated with cytochalasin D will still be able to
| back 89 Answer: E |
front 90 When a potassium ion (K+) moves from the soil into the vacuole of a cell on the surface of a root, it must pass through several cellular structures. Which of the following correctly describes the order in which these structures will be encountered by the ion?
| back 90 Answer: C |
front 91 A mutation that disrupts the ability of an animal cell to add polysaccharide modifications to proteins would most likely cause defects in its
| back 91 Answer: D |
front 92 ECM proteins are made by ribosomes in which part of a eukaryotic cell?
| back 92 Answer: E |
front 93 What types of proteins are not synthesized in the rough ER?
| back 93 Answer: D |
front 94 A biologist ground up some plant leaf cells and then centrifuged the mixture to fractionate the organelles. Organelles in one of the heavier fractions could produce ATP in the light, whereas organelles in the lighter fraction could produce ATP in the dark. The heavier and lighter fractions are most likely to contain, respectively,
| back 94 Answer: D |
front 95 Which structure is not part of the endomembrane system?
| back 95 Answer: B |
front 96 Which structure-function pair is mismatched?
| back 96 Answer: E |
front 97 Cyanide binds with at least one molecule involved in producing ATP. If a cell is exposed to cyanide, most of the cyanide will be found within the
| back 97 Answer: A |
front 98 What is the most likely pathway taken by a newly synthesized protein that will be secreted by a cell?
| back 98 Answer: D |
front 99 Who was/were the first to propose that cell membranes are phospholipid bilayers?
| back 99 Answer: E |
front 100 Singer and Nicolson's fluid mosaic model of the membrane proposed that
| back 100 Answer: D |
front 101 The presence of cholesterol in the plasma membranes of some animals
| back 101 Answer: A |
front 102 According to the fluid mosaic model of cell membranes, which of the following is a true statement about membrane phospholipids?
| back 102 Answer: A |
front 103 Which of the following is one of the ways that the membranes of winter wheat are able to remain fluid when it is extremely cold?
| back 103 Answer: A |
front 104 In order for a protein to be an integral membrane protein it would have to be
| back 104 Answer: C |
front 105 Which of the following is a reasonable explanation for why unsaturated fatty acids help keep any membrane more fluid at lower temperatures?
| back 105 Answer: A |
front 106 Which of the following is true of integral membrane proteins?
| back 106 Answer: C |
front 107 The primary function of polysaccharides attached to the glycoproteins and glycolipids of animal cell membranes is
| back 107 Answer: E |
front 108 In a paramecium, cell surface integral membrane proteins are synthesized
| back 108 Answer: C |
front 109 Which of the following is true of the evolution of cell membranes?
| back 109 Answer: D |
front 110 What kinds of molecules pass through a cell membrane most easily?
| back 110 Answer: B |
front 111 Which of the following is a characteristic feature of a carrier protein in a plasma membrane?
| back 111 Answer: B |
front 112 Nitrous oxide gas molecules diffusing across a cell's plasma membrane is an example of
| back 112 Answer: A |
front 113 Which of the following statements is correct about diffusion?
| back 113 Answer: C |
front 114 Celery stalks that are immersed in fresh water for several hours become stiff and hard. Similar stalks left in a 0.15 M salt solution become limp and soft. From this we can deduce that the cells of the celery stalks are
| back 114 Answer: C |
front 115 Mammalian blood contains the equivalent of 0.15 M NaCl. Seawater contains the equivalent of 0.45 M NaCl. What will happen if red blood cells are transferred to seawater?
| back 115 Answer: A |
front 116 Which of the following statements correctly describes the normal tonicity conditions for typical plant and animal cells?
| back 116 Answer: D |
front 117 When a plant cell, such as one from a peony stem, is submerged in a very hypotonic solution, what is likely to occur?
| back 117 Answer: E |
front 118 Which of the following membrane activities require energy from ATP hydrolysis?
| back 118 Answer: C |
front 119 The phosphate transport system in bacteria imports phosphate into the cell even when the concentration of phosphate outside the cell is much lower than the cytoplasmic phosphate concentration. Phosphate import depends on a pH gradient across the membrane–more acidic outside the cell than inside the cell. Phosphate transport is an example of
| back 119 Answer: E |
front 120 Glucose diffuses slowly through artificial phospholipid bilayers. The cells lining the small intestine, however, rapidly move large quantities of glucose from the glucose-rich food into their glucose-poor cytoplasm. Using this information, which transport mechanism is most probably functioning in the intestinal cells?
| back 120 Answer: E |
front 121 In most cells, there are electrochemical gradients of many ions across the plasma membrane even though there are usually only one or two electrogenic pumps present in the membrane. The gradients of the other ions are most likely accounted for by
| back 121 Answer: A |
front 122 The sodium-potassium pump is called an electrogenic pump because it
| back 122 Answer: C |
front 123 Which of the following is most likely true of a protein that cotransports glucose and sodium ions into the intestinal cells of an animal?
| back 123 Answer: E |
front 124 The movement of potassium into an animal cell requires
| back 124 Answer: C |
front 125 Ions diffuse across membranes through specific ion channels
| back 125 Answer: D |
front 126 Which of the following would increase the electrochemical potential across a membrane?
| back 126 Answer: C |
front 127 The sodium-potassium pump in animal cells requires cytoplasmic ATP to pump ions across the plasma membrane. When the proteins of the pump are first synthesized in the rough ER, what side of the ER membrane will the ATP binding site be on?
| back 127 Answer: A |
front 128 Proton pumps are used in various ways by members of every domain of organisms: Bacteria, Archaea, and Eukarya. What does this most probably mean?
| back 128 Answer: B |
front 129 The difference between pinocytosis and receptor-mediated endocytosis is that
| back 129 Answer: C |
front 130 In receptor-mediated endocytosis, receptor molecules initially project to the outside of the cell. Where do they end up after endocytosis?
| back 130 Answer: C |
front 131 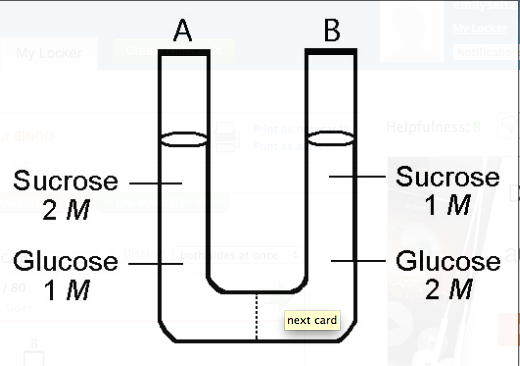 The solutions in the two arms of this U-tube are separated by a membrane that is permeable to water and glucose but not to sucrose. Side A is half-filled with a solution of 2 M sucrose and 1 M glucose. Side B is half-filled with 1 M sucrose and 2 M glucose. Initially, the liquid levels on both sides are equal.
| back 131 Answer: C |
front 132 ***After the system reaches equilibrium, what changes are observed?
| back 132 Answer: C |
front 133 In the small airways of the lung, a thin layer of liquid is needed between the epithelial cells and the mucus layer in order for cilia to beat and move the mucus and trapped particles out of the lung. One hypothesis is that the volume of this airway surface liquid is regulated osmotically by transport of sodium and chloride ions across the epithelial cell membrane. How would the lack of a functional chloride channel in cystic fibrosis patients affect sodium ion transport and the volume of the airway surface liquid?
| back 133 Answer: C |
front 134 A patient has had a serious accident and lost a lot of blood. In an attempt to replenish body fluids, distilled water–equal to the volume of blood lost–is transferred directly into one of his veins. What will be the most probable result of this transfusion?
| back 134 Answer: C |
front 135 In what way do the membranes of a eukaryotic cell vary?
| back 135 Answer: B |
front 136 According to the fluid mosaic model of membrane structure, proteins of the membrane are mostly
| back 136 Answer: C |
front 137 Which of the following factors would tend to increase membrane fluidity?
| back 137 Answer: A |
front 138 Which of the following processes includes all others?
| back 138 Answer: D |
front 139 When chemical, transport, or mechanical work is done by an organism, what happens to the heat generated?
| back 139 Answer: D |
front 140 When ATP releases some energy, it also releases inorganic phosphate. What purpose does this serve (if any) in the cell?
| back 140 Answer:D |
front 141 A number of systems for pumping ions across membranes are powered by ATP. Such ATP-powered pumps are often called ATPases although they don't often hydrolyze ATP unless they are simultaneously transporting ions. Because small increases in calcium ions in the cytosol can trigger a number of different intracellular reactions, cells keep the cytosolic calcium concentration quite low under normal conditions, using ATP-powered calcium pumps. For example, muscle cells transport calcium from the cytosol into the membranous system called the sarcoplasmic reticulum (SR). If a resting muscle cell's cytosol has a free calcium ion concentration of 10⁻⁷ while the concentration in the SR is 10⁻², then how is the ATPase acting?
| back 141 Answer: C |
front 142 What is the difference (if any) between the structure of ATP and the structure of the precursor of the A nucleotide in RNA?
| back 142 Answer: E |
front 143 The active site of an enzyme is the region that
| back 143 Answer: B |
front 144 According to the induced fit hypothesis of enzyme catalysis, which of the following is correct?
| back 144 Answer: D |
front 145 Mutations that result in single amino acid substitutions in an enzyme
| back 145 Answer: D |
front 146 Increasing the substrate concentration in an enzymatic reaction could overcome which of the following?
| back 146 Answer: C |
front 147 Zinc, an essential trace element for most organisms, is present in the active site of the enzyme carboxypeptidase. The zinc most likely functions as a(n)
| back 147 Answer: D |
front 148 In order to attach a particular amino acid to the tRNA molecule that will transport it, an enzyme, an aminoacyl-tRNA synthetase, is required, along with ATP. Initially, the enzyme has an active site for ATP and another for the amino acid, but it is not able to attach the tRNA. What must occur in order for the final attachment to occur?
| back 148 Answer: B |
front 149 Some of the drugs used to treat HIV patients are competitive inhibitors of the HIV reverse transcriptase enzyme. Unfortunately, the high mutation rate of HIV means that the virus rapidly acquires mutations with amino acid changes that make them resistant to these competitive inhibitors. Where in the reverse transcriptase enzyme would such amino acid changes most likely occur in drug-resistant viruses?
| back 149 Answer: A |
front 150 Protein kinases are enzymes that transfer the terminal phosphate from ATP to an amino acid residue on the target protein. Many are located on the plasma membrane as integral membrane proteins or peripheral membrane proteins. What purpose may be served by their plasma membrane localization?
| back 150 Answer: B |
front 151 How does a noncompetitive inhibitor decrease the rate of an enzyme reaction?
| back 151 Answer: B |
front 152 The mechanism in which the end product of a metabolic pathway inhibits an earlier step in the pathway is most precisely described as
| back 152 Answer: B |
front 153 Which of the following statements describes enzyme cooperativity?
| back 153 Answer: C |
front 154 Allosteric enzyme regulation is usually associated with
| back 154 Answer: D |
front 155 Which of the following is an example of cooperativity?
| back 155 Answer: C |
front 156 Protein kinases are enzymes that catalyze phosphorylation of target proteins at specific sites, whereas protein phosphatases catalyze removal of phosphate(s) from phosphorylated proteins. Phosphorylation and dephosphorylation can function as an on-off switch for a protein's activity, most likely through
| back 156 Answer: A |
front 157 Besides turning enzymes on or off, what other means does a cell use to control enzymatic activity?
| back 157 Answer: B |
front 158 In experimental tests of enzyme evolution, where a gene encoding an enzyme is subjected to multiple cycles of random mutagenesis and selection for altered substrate specificity, the resulting enzyme had multiple amino acid changes associated with altered substrate specificity. Where in the enzyme were these amino acid changes located?
| back 158 Answer: C |
front 159 How might an amino acid change at a site distant from the active site of the enzyme alter the enzyme's substrate specificity?
| back 159 Answer: C |
front 160 Succinate dehydrogenase catalyzes the conversion of succinate to fumarate. The reaction is inhibited by malonic acid, which resembles succinate but cannot be acted upon by succinate dehydrogenase. Increasing the ratio of succinate to malonic acid reduces the inhibitory effect of malonic acid.
| back 160 Answer: C |
front 161 Succinate dehydrogenase catalyzes the conversion of succinate to fumarate. The reaction is inhibited by malonic acid, which resembles succinate but cannot be acted upon by succinate dehydrogenase. Increasing the ratio of succinate to malonic acid reduces the inhibitory effect of malonic acid.
| back 161 Answer: A |
front 162 A series of enzymes catalyze the reaction X → Y → Z → A. Product A binds to the enzyme that converts X to Y at a position remote from its active site. This binding decreases the activity of the enzyme.
| back 162 Answer: C |
front 163 A series of enzymes catalyze the reaction X → Y → Z → A. Product A binds to the enzyme that converts X to Y at a position remote from its active site. This binding decreases the activity of the enzyme.
| back 163 Answer: B |
front 164 Choose the pair of terms that correctly completes this sentence: Catabolism is to anabolism as ________ is to ________.
| back 164 ANswer: B |
front 165 What is the term for metabolic pathways that release stored energy by breaking down complex molecules?
| back 165 Answer: B |
front 166 Why does the oxidation of organic compounds by molecular oxygen to produce CO₂ and water release free energy?
| back 166 Answer: B |
front 167 Which of the following statements describes NAD⁺?
| back 167 Answer: A |
front 168 The oxygen consumed during cellular respiration is involved directly in which process or event?
| back 168 Answer: B |
front 169 An electron loses potential energy when it
| back 169 Answer: B |
front 170 The transport of pyruvate into mitochondria depends on the proton-motive force across the inner mitochondrial membrane. How does pyruvate enter the mitochondrion?
| back 170 Answer: A |
front 171 During cellular respiration, acetyl CoA accumulates in which location?
| back 171 Answer: E |
front 172 A young animal has never had much energy. He is brought to a veterinarian for help and is sent to the animal hospital for some tests. There they discover his mitochondria can use only fatty acids and amino acids for respiration, and his cells produce more lactate than normal. Of the following, which is the best explanation of his condition?
| back 172 Answer: A |
front 173 During aerobic respiration, electrons travel downhill in which sequence?
| back 173 Answer: B |
front 174 Where are the proteins of the electron transport chain located?
| back 174 Answer: C |
front 175 In cellular respiration, the energy for most ATP synthesis is supplied by
| back 175 Answer: B |
front 176 During aerobic respiration, which of the following directly donates electrons to the electron transport chain at the lowest energy level?
| back 176 Answer: E |
front 177 Inside an active mitochondrion, most electrons follow which pathway?
| back 177 Answer: E |
front 178 In chemiosmotic phosphorylation, what is the most direct source of energy that is used to convert ADP + Pi to ATP?
| back 178 Answer: D |
front 179 Energy released by the electron transport chain is used to pump H⁺ into which location in eukaryotic cells?
| back 179 Answer: D |
front 180 The direct energy source that drives ATP synthesis during respiratory oxidative phosphorylation in eukaryotic cells is
| back 180 Answer: D |
front 181 When hydrogen ions are pumped from the mitochondrial matrix across the inner membrane and into the intermembrane space, the result is the
| back 181 Answer: D |
front 182 Chemiosmotic ATP synthesis (oxidative phosphorylation) occurs in
| back 182 Answer: D |
front 183 What is proton-motive force?
| back 183 Answer: B |
front 184 Phosphofructokinase is an important control enzyme in the regulation of cellular respiration. Which of the following statements correctly describes phosphofructokinase activity?
| back 184 Answer: E |
front 185 Phosphofructokinase is an allosteric enzyme that catalyzes the conversion of fructose 6-phosphate to fructose 1,6-bisphosphate, an early step of glycolysis. In the presence of oxygen, an increase in the amount of ATP in a cell would be expected to
| back 185 Answer: A |
front 186 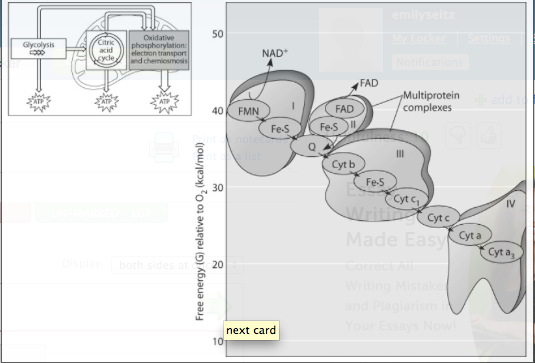 igure 9.3 shows the electron transport chain. Which of the following is the combination of substances that is initially added to the chain?
| back 186 Answer: D |
front 187 ***Which of the following most accurately describes what is happening along the electron transport chain in Figure 9.3?
| back 187 Answer: B |
front 188 ***Which of the protein complexes labeled with Roman numerals in Figure 9.3 will transfer electrons to O₂?
| back 188 Answer: D |
front 189 What happens at the end of the chain in Figure 9.3?
| back 189 Answer: C |
front 190 Exposing inner mitochondrial membranes to ultrasonic vibrations will disrupt the membranes. However, the fragments will reseal "inside out." These little vesicles that result can still transfer electrons from NADH to oxygen and synthesize ATP. If the membranes are agitated further, however, the ability to synthesize ATP is lost.
| back 190 Answer: A |
front 191 The immediate energy source that drives ATP synthesis by ATP synthase during oxidative phosphorylation is the
| back 191 Answer: D |
front 192 The final electron acceptor of the electron transport chain that functions in aerobic oxidative phosphorylation is
| back 192 Answer: A |
front 193 When electrons flow along the electron transport chains of mitochondria, which of the following changes occurs?
| back 193 Answer: A |
front 194 Which of the following are products of the light reactions of photosynthesis that are utilized in the Calvin cycle?
| back 194 Answer: E |
front 195 Where does the Calvin cycle take place?
| back 195 Answer: A |
front 196 In autotrophic bacteria, where are the enzymes located that can carry on carbon fixation (reduction of carbon dioxide to carbohydrate)?
| back 196 Answer: C |
front 197 When oxygen is released as a result of photosynthesis, it is a direct by-product of
| back 197 Answer: B |
front 198 In the thylakoid membranes, what is the main role of the antenna pigment molecules?
| back 198 Answer: B |
front 199 Which of the events listed below occurs in the light reactions of photosynthesis?
| back 199 Answer: E |
front 200 Which statement describes the functioning of photosystem II?
| back 200 Answer: D |
front 201 Which of the following are directly associated with photosystem I?
| back 201 Answer: B |
front 202 Some photosynthetic organisms contain chloroplasts that lack photosystem II, yet are able to survive. The best way to detect the lack of photosystem II in these organisms would be
| back 202 Answer: B |
front 203 What are the products of linear photophosphorylation?
| back 203 Answer: C |
front 204 As a research scientist, you measure the amount of ATP and NADPH consumed by the Calvin cycle in 1 hour. You find 30,000 molecules of ATP consumed, but only 20,000 molecules of NADPH. Where did the extra ATP molecules come from?
| back 204 Answer: C |
front 205 Assume a thylakoid is somehow punctured so that the interior of the thylakoid is no longer separated from the stroma. This damage will have the most direct effect on which of the following processes?
| back 205 Answer: D |
front 206 What does the chemiosmotic process in chloroplasts involve?
| back 206 Answer: A |
front 207 In mitochondria, chemiosmosis translocates protons from the matrix into the intermembrane space, whereas in chloroplasts, chemiosmosis translocates protons from
| back 207 Answer: C |
front 208 Where are the molecules of the electron transport chain found in plant cells?
| back 208 Answer: A |
front 209 Reduction of NADP⁺ occurs during
| back 209 Answer:A |
front 210 The splitting of carbon dioxide to form oxygen gas and carbon compounds occurs during
| back 210 Answer: D |
front 211 P680⁺ is said to be the strongest biological oxidizing agent. Why?
| back 211 Answer: D |
front 212 Some photosynthetic bacteria (e.g., purple sulfur bacteria) have only photosystem I, whereas others (e.g., cyanobacteria) have both photosystem I and photosystem II. Which of the following might this observation imply?
| back 212 ANswer: B |
front 213 electron flow may be photoprotective (protective to light-induced damage). Which of the following experiments could provide information on this phenomenon?
| back 213 Answer: A |
front 214 In metabolic processes of cell respiration and photosynthesis, prosthetic groups such as heme and iron-sulfur complexes are encountered in components of the electron transport chain. What do they do?
| back 214 ANswer: E |
front 215 In a cyanobacterium, the reactions that produce NADPH occur in
| back 215 Answer: A |
front 216 The reactions that produce molecular oxygen (O₂) take place in
| back 216 Answer: A |
front 217 The accumulation of free oxygen in Earth's atmosphere began
| back 217 Answer: C |
front 218 A flask containing photosynthetic green algae and a control flask containing water with no algae are both placed under a bank of lights, which are set to cycle between 12 hours of light and 12 hours of dark. The dissolved oxygen concentrations in both flasks are monitored. Predict what the relative dissolved oxygen concentrations will be in the flask with algae compared to the control flask.
| back 218 Answer: D |
front 219 Where do the enzymatic reactions of the Calvin cycle take place?
| back 219 Answer: A |
front 220 What is the primary function of the Calvin cycle?
| back 220 Answer: E |
front 221 The NADPH required for the Calvin cycle comes from
| back 221 Answer: A |
front 222 Reactions that require CO₂ take place in
| back 222 Answer: B |
front 223 Which of the following statements best represents the relationships between the light reactions and the Calvin cycle?
| back 223 Answer: A |
front 224 Three "turns" of the Calvin cycle generate a "surplus" molecule of glyceraldehyde 3-phosphate (G3P). Which of the following is a consequence of this?
| back 224 Answer: D |
front 225 In the process of carbon fixation, RuBP attaches a CO₂ to produce a six-carbon molecule, which is then split to produce two molecules of 3-phosphoglycerate. After phosphorylation and reduction produces glyceraldehyde 3-phosphate (G3P), what more needs to happen to complete the Calvin cycle?
| back 225 ANswer: D |
front 226 The phylogenetic distribution of the enzyme rubisco is limited to
| back 226 Answer: D |
front 227 Photorespiration occurs when rubisco reacts RuBP with
| back 227 ANswer: B |
front 228 Plants photosynthesize only in the light. Plants respire
| back 228 Answer: C |
front 229 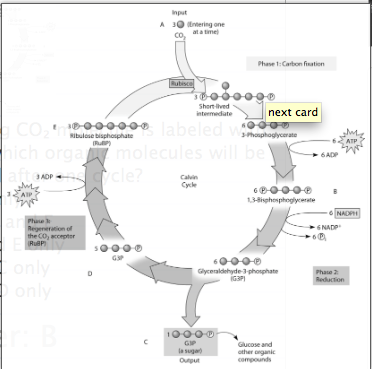 If the carbon atom of the incoming CO₂ molecule is labeled with a radioactive isotope of carbon, which organic molecules will be radioactively labeled after one cycle?
| back 229 Answer: B |
front 230 ***If ATP used by this plant is labeled with radioactive phosphorus, which molecule or molecules of the Calvin cycle will be radioactively labeled first?
| back 230 Answer: D |
front 231 ***Which molecule(s) of the Calvin cycle is (are) also found in glycolysis?
| back 231 Answer: D |
front 232 To identify the molecule that accepts CO₂, Calvin and Benson manipulated the carbon-fixation cycle by either cutting off CO₂ or cutting off light from cultures of photosynthetic algae. They then measured the concentrations of various metabolites immediately following the manipulation. How would these experiments help identify the CO₂ acceptor? Study Figure 10.2 to help you in determining the correct answer.
| back 232 Answer: C |
front 233 The light reactions of photosynthesis supply the Calvin cycle with
| back 233 Answer: D |
front 234 Which of the following sequences correctly represents the flow of electrons during photosynthesis?
| back 234 Answer: B |
front 235 Which of the following does not occur during the Calvin cycle?
| back 235 Answer: C |
front 236 Which process is most directly driven by light energy?
| back 236 Answer:D |