Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
infectious diseases manifesting in the gastrointestinal tract
front 1 dental caries disease table | back 1 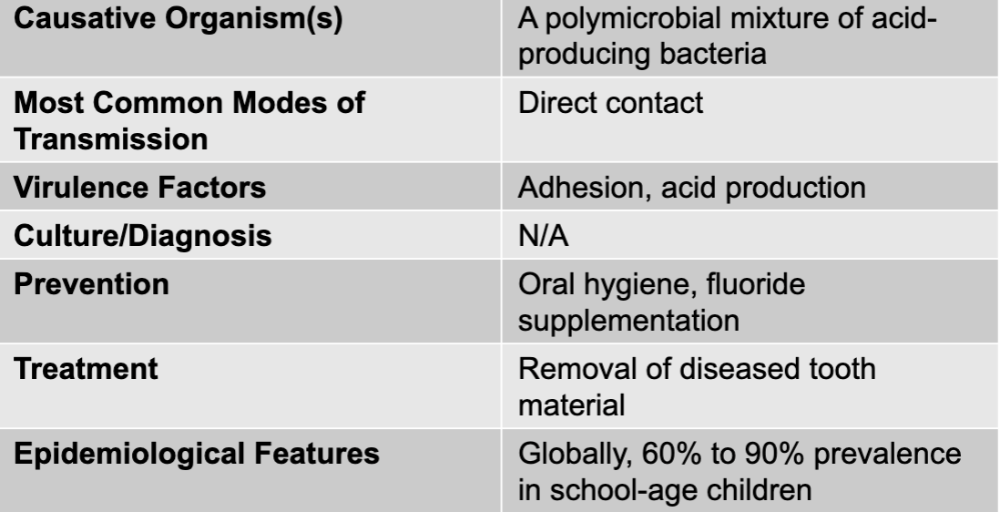 |
front 2 dental caries causative agent | back 2 a polymicrobial mixture of acid-producing bacteria |
front 3 dental caries mode of transmission | back 3 direct contact |
front 4 dental caries virulence factors | back 4 adhesion, acid production |
front 5 dental caries culture/diagnosis | back 5 N/A |
front 6 dental caries prevention | back 6 oral hygiene, fluoride supplementation |
front 7 dental caries treatment | back 7 removal of diseased tooth material |
front 8 dental caries epidemiological features | back 8 globally, 60% to 90% prevalence in school-age children |
front 9 periodontitis disease table | back 9 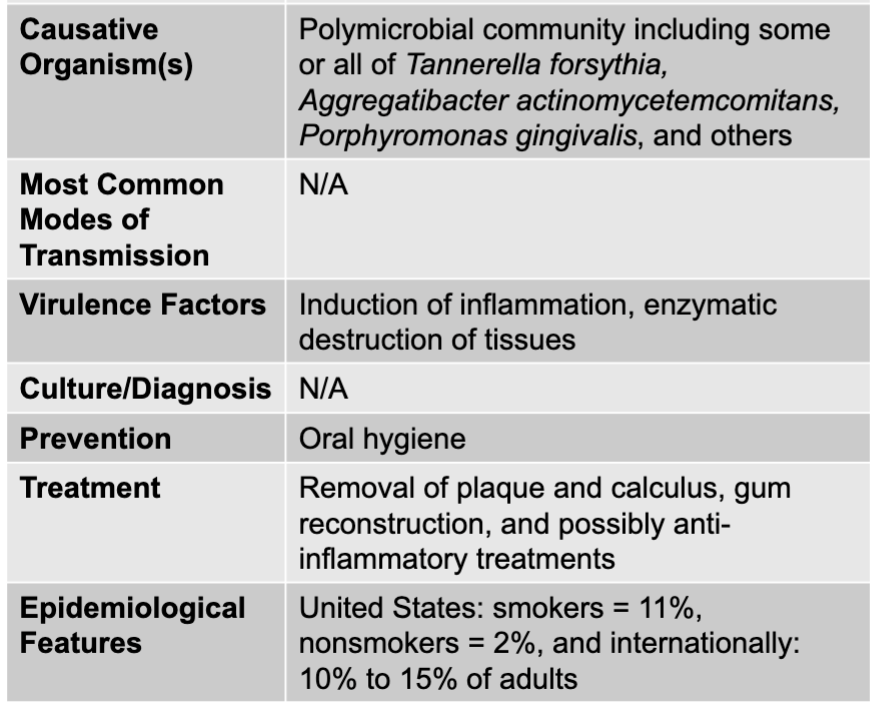 |
front 10 necrotizing ulcerative gingivitis and periodontitis disease table | back 10 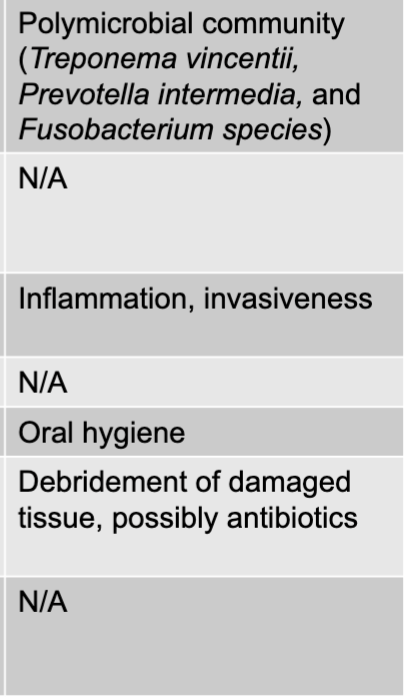 |
front 11 periodontitis causative agents | back 11 polymicrobial community including some or all of Tannerella forsythia, Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, and others |
front 12 periodontitis virulence factors | back 12 induction of inflammation, enzymatic destruction of tissues |
front 13 periodontitis prevention | back 13 oral hygiene |
front 14 periodontitis treatment | back 14 removal of plaque and calculus, gum reconstruction, and possibly anti-inflammatory treatments |
front 15 periodontitis epidemiological features | back 15 US: smokers = 11%, nonsmokers = 2%, and internationally: 10% to 15% of adults |
front 16 NUG or NUP causative agents | back 16 polymicrobial community (treponema vincentii, prevotella intermedia, and fuso bacterium species) |
front 17 NUG or NUP virulence factors | back 17 inflammation, invasiveness |
front 18 NUG or NUP prevention | back 18 oral hygiene |
front 19 NUG or NUP treatment | back 19 debridement of damaged tissue, possibly antibiotics |
front 20 NUG or NUP epidemiological features | back 20 N/A |
front 21 mumps disease table | back 21 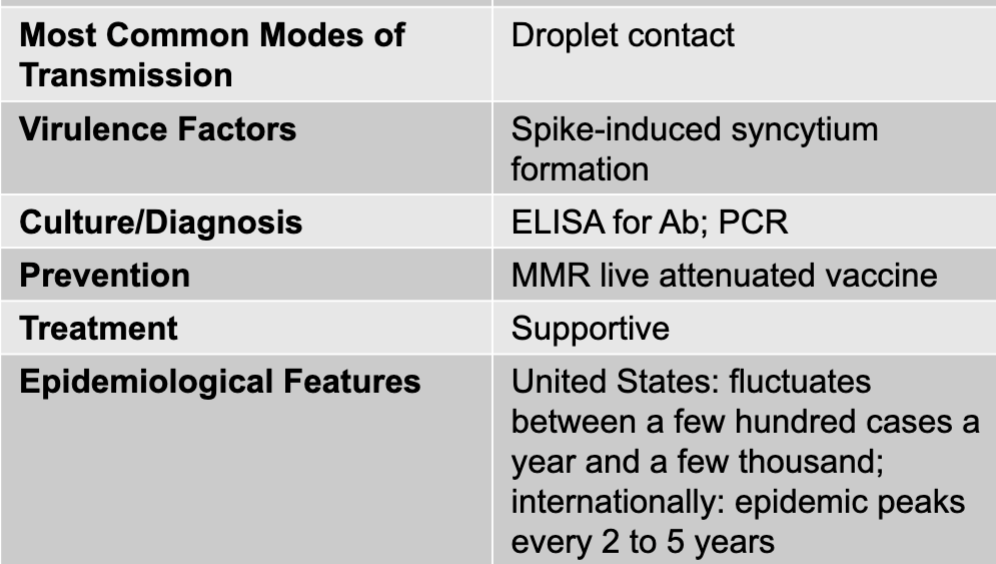 |
front 22 mumps causative agent | back 22 mumps virus (genus paramyxovirus) |
front 23 mumps mode of transmission | back 23 droplet contact |
front 24 mumps virulence factors | back 24 spike-induced syncytium formation |
front 25 mumps culture/diagnosis | back 25 ELISA for Ab; PCR |
front 26 mumps prevention | back 26 MMR live attenuated vaccine |
front 27 mumps treatment | back 27 supportive |
front 28 mumps epidemiological features | back 28 US: fluctuates between a few hundred cases a year and a few thousand; internationally; epidemic peaks every 2 to 5 years |
front 29 gastritis and gastric ulcers disease table | back 29 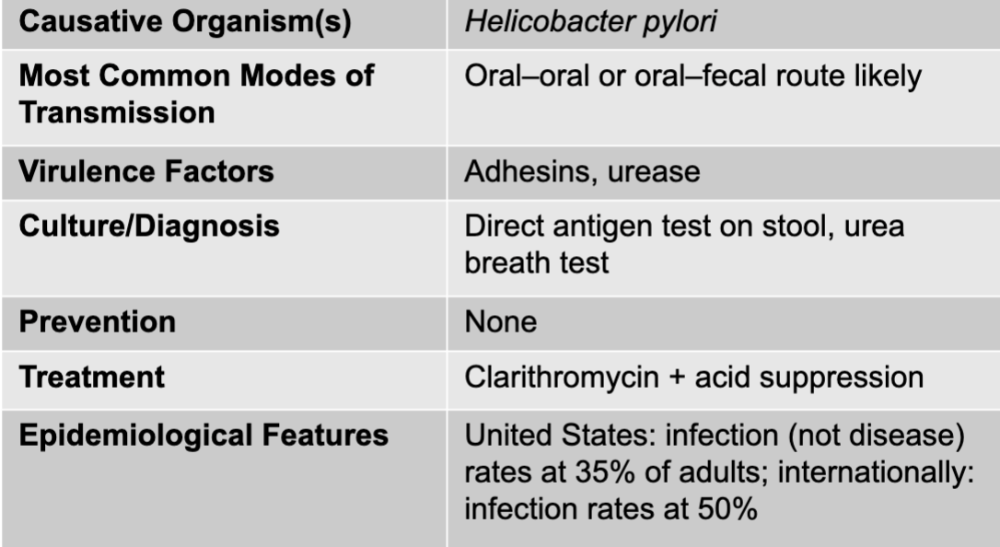 |
front 30 gastritis causative agents | back 30 helicobacter pylori |
front 31 gastritis mode of transmission | back 31 oral-oral or oral-fecal route likely |
front 32 gastritis virulence factors | back 32 adhesins, urease |
front 33 gastritis culture/diagnosis | back 33 direct antigen test on stool, urea breath test |
front 34 gastritis prevention | back 34 none |
front 35 gastritis treatment | back 35 clarithromycin + acid suppression |
front 36 gastritis epidemiological features | back 36 US: infection (not disease) rates at 35% of adults; internationally: infection rates at 50% |
front 37 acute diarrhea causative agents | back 37 salmonella, shigella, shiga toxin-producing E. coli, other E. colie, campylobacter, clostridioides difficile, vibrio cholerae, and non-cholera vibrio species |
front 38 salmonella disease table | back 38 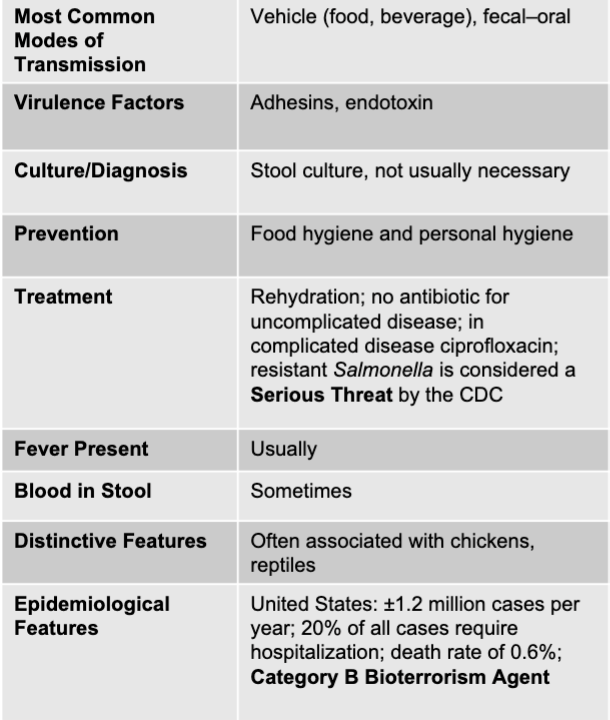 |
front 39 shigella disease table | back 39 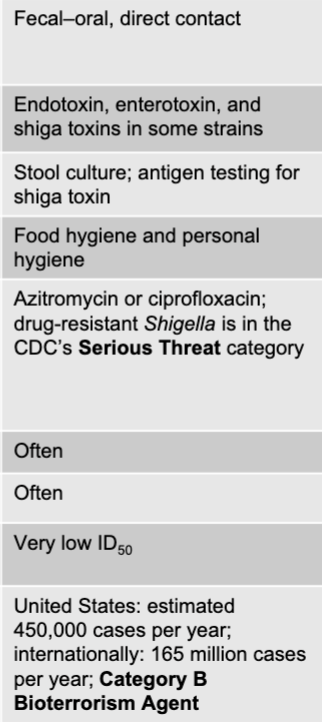 |
front 40 shiga toxin-producing E. coli disease table | back 40 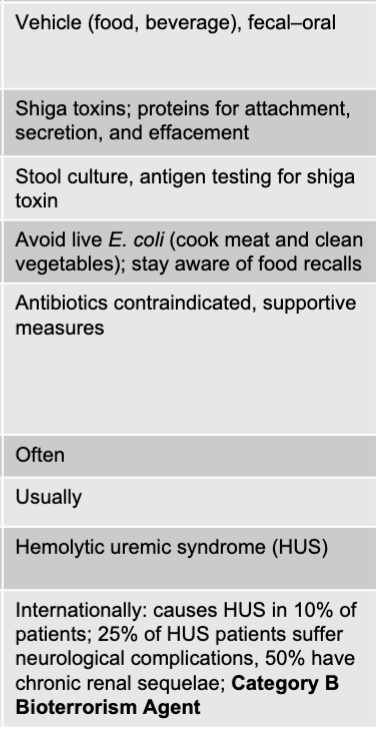 |
front 41 other E. coli disease table | back 41 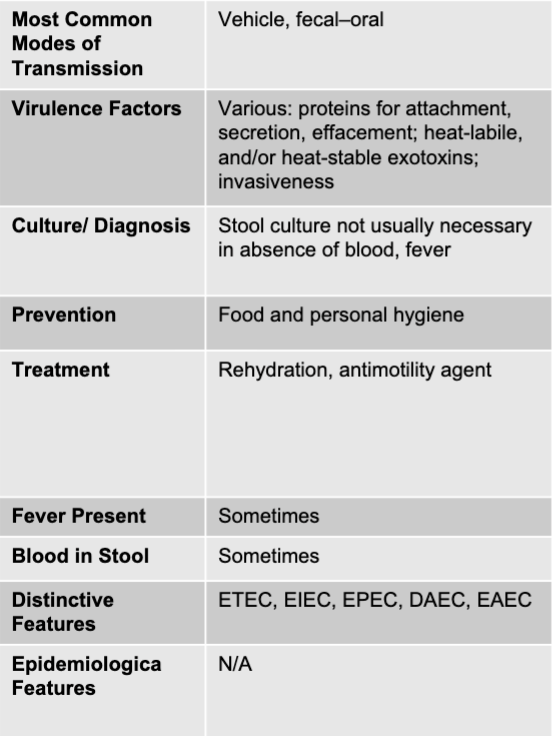 |
front 42 campylobacter disease table | back 42  |
front 43 clostridioides difficile disease table | back 43  |
front 44 vibrio cholerae disease table | back 44 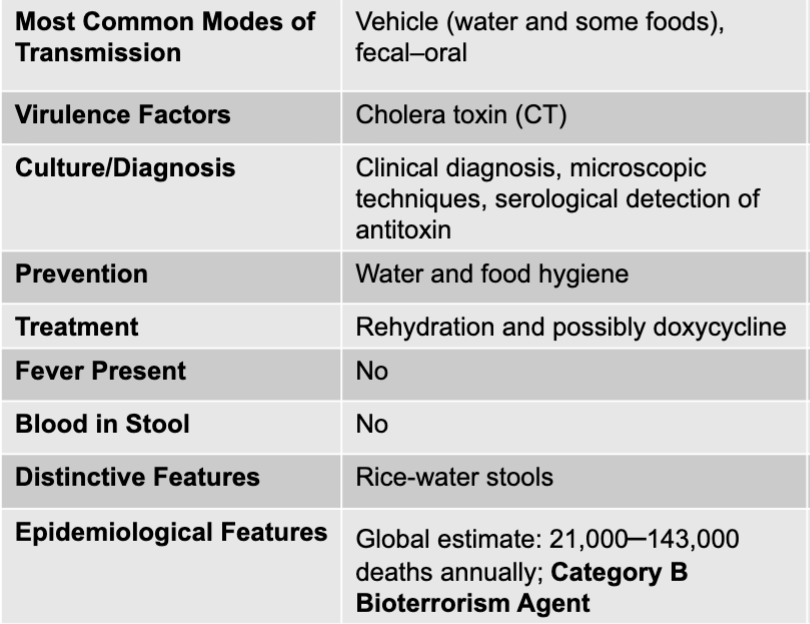 |
front 45 non-cholera vibrio species | back 45 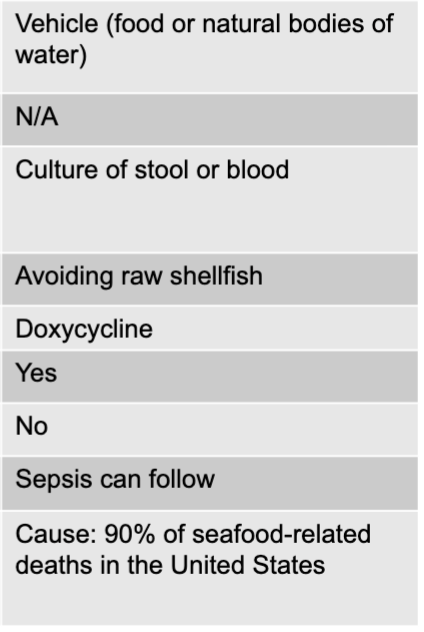 |
front 46 salmonella mode of transmission | back 46 vehicle (food, beverage), fecal-oral |
front 47 salmonella virulence factors | back 47 adhesions, endotoxin |
front 48 salmonella culture/diagnosis | back 48 stool culture, not usually necessary |
front 49 salmonella prevention | back 49 food hygiene and personal hygiene |
front 50 salmonella treatment | back 50 rehydration; no antibiotic for uncomplicated disease; in complicated disease ciprofloxacin; resistant salmonella is considered a serious threat by the CDC |
front 51 is fever present with salmonella? | back 51 usually |
front 52 is blood present in the stool with salmonella? | back 52 sometimes |
front 53 salmonella distinctive features | back 53 often associated with chickens, reptiles |
front 54 salmonella epidemiological features | back 54 US: +/- 1.2 million cases per year; 20% of all cases require hospitalization; death rate of 0.6%; category b bioterrorism agent |
front 55 shigella mode of transmission | back 55 fecal-oral, direct contact |
front 56 shigella virulence factors | back 56 endotoxin, enterotoxinm and shiga toxins in some strains |
front 57 shigella culture/diagnosis | back 57 stool culture; antigen testing for shiga toxin |
front 58 shigella prevention | back 58 food hygiene and personal hygiene |
front 59 shigella treatment | back 59 azitromycin or ciprofloxacin; drug-resistant shigella is in the CDC's serious threat category |
front 60 is fever present with shigella? | back 60 often |
front 61 is blood in the stool with shigella? | back 61 often |
front 62 shigella distinctive features | back 62 very low in ID50 |
front 63 shigella epidemiological features | back 63 US: estimated 450,000 cases per year; internationally: 165 million cases per year; category b bioterrorism agent |
front 64 shiga toxin-producing E. coli mode of transmission | back 64 vehicle (food, beverage), fecal-oral |
front 65 shiga toxin-producing E.coli virulence factors | back 65 shiga toxins; proteins for attachment, secretion, and effacement |
front 66 shiga toxin-producing culture/diagnosis | back 66 stool culture, antigen testing for shiga toxin |
front 67 shiga-toxin producing E.coli prevention | back 67 avoid live E. coli (cook meat and clean vegetables); stay aware of food recalls |
front 68 shiga-toxin producing E.coli treatment | back 68 antibiotics contraindicated, supportive measures |
front 69 is fever present when shiga-toxin producing E.coli? | back 69 often |
front 70 is blood in the stool with shiga-toxin producing E.coli? | back 70 usually |
front 71 shiga toxin-producing distinctive features | back 71 hemolytic uremic syndrome |
front 72 shiga toxin-producing epidemiological features | back 72 internationally: causes HUS in 10% of patients; 25% of HUS patients suffer neurological complications, 50% have chronic renal sequelae; category b bioterrorism agent |
front 73 other E.coli mode of transmission | back 73 vehicle, fecal-oral |
front 74 other E.coli virulence factors | back 74 various: proteins for attachment, secretion, effacement; heat-liable, and/or heat-stable exotoxins; invasiveness |
front 75 other E.coli culture/diagnosis | back 75 stool culture not usually necessary in absence of blood, fever |
front 76 other E.coli prevention | back 76 food and personal hygiene |
front 77 other E.coli treatment | back 77 rehydration, anti-motility agent |
front 78 other E.coli fever present | back 78 sometimes |
front 79 other E.coli blood in stool | back 79 sometimes |
front 80 other E.coli distinctive features | back 80 ETEC, EIEC, EPEC, DAEC, EAEC |
front 81 campylobacter mode of transmission | back 81 vehicle (food, water), fecal-oral |
front 82 campylobacter virulence factors | back 82 adhesins, exotoxin, and induction of autoimmunity |
front 83 campylobacter culture/diagnosis | back 83 stool culture not usually necessary; dark-field microscopy |
front 84 campylobacter prevention | back 84 food and personal hygiene |
front 85 campylobacter treatment | back 85 rehydration; azithromycin in severe cases |
front 86 campylobacter fever present | back 86 usually |
front 87 campylobacter blood in stool | back 87 no |
front 88 campylobacter distinctive features | back 88 guillain-barre syndrome |
front 89 campylobacter epidemiolgica features | back 89 US: 1.3 million cases per year; internationally: 400 million cases per year |
front 90 clostridioides difficle mode of transmission | back 90 endogenous (normal biota) |
front 91 clostridioides difficle virulence factors | back 91 enterotoxins A and B |
front 92 clostridioides difficle culture/diagnosis | back 92 stool culture, PCR, ELISA demonstration of toxins in stool |
front 93 clostridioides difficle prevention | back 93 N/A |
front 94 clostridioides difficle treatment | back 94 metronidazole in mild cases, vancomycin for severe, fecal transplants, resistant strains are in the CDC's urgent threat category |
front 95 clostridioides difficle fever present | back 95 sometimes |
front 96 clostridioides difficle blood in stool | back 96 not usually; mucus prominent |
front 97 clostridioides difficle distinctive features | back 97 associated with disruption of normal biota |
front 98 clostridioides difficle epidemiologic features | back 98 US: 500,000 cases per year |
front 99 vibrio cholerae mode of transmission | back 99 vehicle (water and some foods), fecal-oral |
front 100 vibrio cholerae virulence factors | back 100 cholera toxin |
front 101 vibrio cholerae culture/diagnosis | back 101 clinical diagnosis, microscopic techniques, serological detection of antitoxin |
front 102 vibrio cholerae prevention | back 102 water and food hygiene |
front 103 vibrio cholerae treatment | back 103 rehydration and possibly doxycycline |
front 104 vibrio cholerae fever present | back 104 no |
front 105 vibrio cholerae blood in stool | back 105 no |
front 106 vibrio cholerae distinctive features | back 106 rice-water stools |
front 107 vibrio cholerae epidemiological features | back 107 global estimate: 21,000-143,000 deaths annually; category b bioterrorism agent |
front 108 non-cholera vibrio species mode of transmission | back 108 vehicle (food or natural bodies of water) |
front 109 non-cholera vibrio species virulence factors | back 109 N/A |
front 110 non-cholera vibrio species culture diagnosis | back 110 culture of stool or blood |
front 111 non-cholera vibrio species prevention | back 111 avoiding raw shellfish |
front 112 non-cholera vibrio species treatment | back 112 doxycycline |
front 113 non-cholera vibrio species fever present | back 113 yes |
front 114 non-cholera vibrio species blood in stoool | back 114 no |
front 115 non-cholera vibrio species distinctive features | back 115 sepsis can follow |
front 116 non-cholera vibrio species epidemiological features | back 116 cause: 90% of seafood-related deaths in the US |
front 117 nonbacterial causes of acute diarrhea | back 117 cryptosporidium, rotavirus, norovirus |
front 118 cryptosporidium mode of transmission | back 118 vehicle (water, food), fecal-oral |
front 119 cryptosporidium virulence factors | back 119 intracellular growth |
front 120 cryptosporidium culture/diagnosis | back 120 acid-fast staining, ruling out bacteria |
front 121 cryptosporidium prevention | back 121 water treatment, proper food handling |
front 122 cryptosporidium treatment | back 122 none of nitazoxanide |
front 123 cryptosporidium fever present | back 123 often |
front 124 cryptosporidium blood in stool | back 124 not usually |
front 125 cryptosporidium distinctive features | back 125 resistant to chlorine disinfection |
front 126 cryptosporidium epidemiological features | back 126 US: estimated 748,000 cases per year; 30% seropositive; category b bioterrorism agent |
front 127 rotavirus mode of transmission | back 127 fecal-oral, vehicle, formite |
front 128 rotavirus virulence factors | back 128 N/A |
front 129 rotavirus culture/diagnosis | back 129 rapid antigen test |
front 130 rotavirus prevention | back 130 oral live-virus vaccine |
front 131 rotavirus treatment | back 131 rehydration |
front 132 rotavirus fever present | back 132 often |
front 133 rotavirus blood in stool | back 133 no |
front 134 rotavirus distinctive features | back 134 severe in infants |
front 135 rotavirus epidemiological features | back 135 US: 2-3 million cases per year internationally; 125 million cases of infantile diarrhea annually |
front 136 norovirus mode of transmission | back 136 indirect, vehicle (food), direct contact |
front 137 norovirus virulence factors | back 137 limited immunity to reinfection |
front 138 norovirus culture/diagnosis | back 138 rapid antigen test |
front 139 norovirus prevention | back 139 hygiene |
front 140 norovirus treatment | back 140 rehydration |
front 141 norovirus fever present | back 141 sometimes |
front 142 norovirus blood in stool | back 142 no |
front 143 norovirus distinctive features | back 143 resistant to disinfection |
front 144 norovirus epidemiological features | back 144 US: second most common cause of foodborne illness hospitalizations |
front 145 are fevers present in acute diarrhea with vomitting? | back 145 not usually |
front 146 is blood in stool present in acute diarrhea with vomitting? | back 146 no |
front 147 acute diarrhea with vomiting causative agents | back 147 staphylococcus aureus exotoxin, bacillus cereus exotoxin, clostridium perfringens exotoxin |
front 148 staphylococcus aureus exotoxin mode of transmission | back 148 vehicle (food) |
front 149 staphylococcus aureus exotoxin virulence factors | back 149 heat-stable exotoxin |
front 150 staphylococcus aureus exotoxin culture/diagnosis | back 150 usually based on epidemiological evidence |
front 151 staphylococcus aureus exotoxin prevention | back 151 proper food handling |
front 152 staphylococcus aureus exotoxin treatment | back 152 supportive |
front 153 staphylococcus aureus exotoxin distinctive features | back 153 suspect in foods with high salt or sugar content |
front 154 staphylococcus aureus exotoxin epidemiological features | back 154 US: estimated 240,000 cases per year; category b bioterrorism agent |
front 155 bacillus cereus exotoxin mode of transmission | back 155 vehicle (food) |
front 156 bacillus cereus exotoxin virulence factors | back 156 heat-stable toxin, heat-liable toxin |
front 157 bacillus cereus exotoxin culture/diagnosis | back 157 microscopic analysis of food or stool |
front 158 bacillus cereus exotoxin prevention | back 158 proper food handling |
front 159 bacillus cereus exotoxin treatment | back 159 supportive |
front 160 bacillus cereus exotoxin distinctive features | back 160 two forms: emetic and diarrheal |
front 161 bacillus cereus exotoxin epidemiological features | back 161 US: estimated 63,000 cases per year |
front 162 clostridium perfringens exotoxin mode of transmission | back 162 vehicle (food) |
front 163 clostridium perfringens exotoxin virulence factors | back 163 heat-liable toxin |
front 164 clostridium perfringens exotoxin culture/diagnosis | back 164 detection of toxin in stool |
front 165 clostridium perfringens exotoxin prevention | back 165 proper food handling |
front 166 clostridium perfringens exotoxin treatment | back 166 supportive |
front 167 clostridium perfringens exotoxin distinctive features | back 167 acute abdominal pain |
front 168 clostridium perfringens exotoxin epidemiological features | back 168 US: estimated 966,000 cases per year, category b bioterrorism agent |
front 169 clostridium perfringens exotoxin disease table | back 169 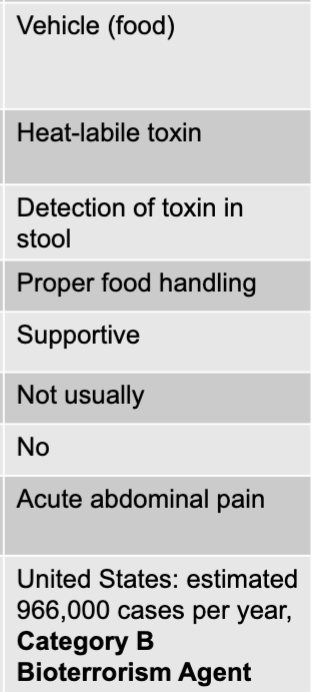 |
front 170 bacillus cereus exotoxin disease table | back 170  |
front 171 staphylococcus aureus exotoxin disease table | back 171 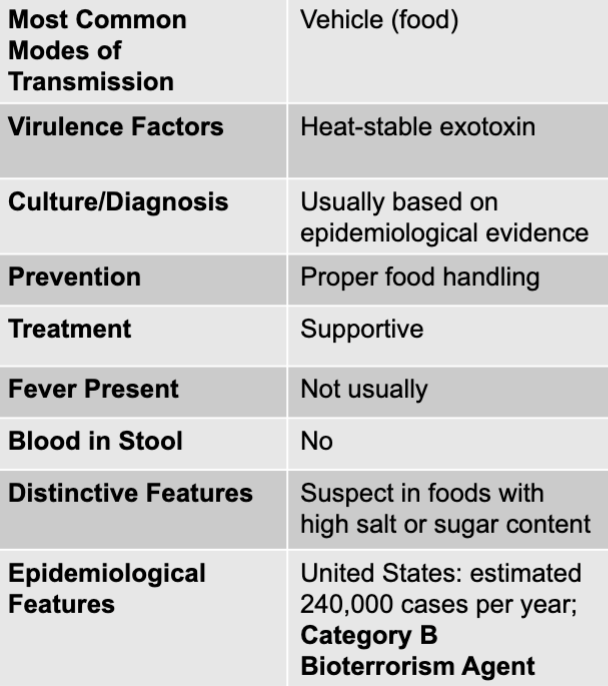 |
front 172 chronic disease causative agents | back 172 enteroaggregative E.coli (EAEC), cyclospora cayetanensis, giardia lamblia, entamoeba histolytica |
front 173 enteroaggregative E.coli (EAEC) mode of transmission | back 173 vehicle (food, water), fecal-oral |
front 174 enteroaggregative E.coli (EAEC) virulence factors | back 174 ? |
front 175 enteroaggregative E.coli (EAEC) | back 175 difficult to distinguish from other E. coli |
front 176 enteroaggregative E.coli (EAEC) treatment | back 176 rehydration of ciprofioxacin |
front 177 enteroaggregative E.coli (EAEC) blood in stool | back 177 sometimes, mucus also |
front 178 enteroaggregative E.coli (EAEC) fever present | back 178 no |
front 179 enteroaggregative E.coli (EAEC) distinctive features | back 179 chronic in the malnourished |
front 180 enteroaggregative E.coli (EAEC) epidemiological features | back 180 developing countries: 87% of chronic diarrhea in children >2 years old |
front 181 cyclospora cayetanensis mode of transmission | back 181 fecal-oral, vehicle |
front 182 cyclospora cayetanensis virulence factors | back 182 invasiveness |
front 183 cyclospora cayetanensis culture/diagnosis | back 183 stool examination, PCR |
front 184 cyclospora cayetanensis prevention | back 184 washing, cooking food, and personal hygiene |
front 185 cyclospora cayetanensis treatment | back 185 TMP-SMZ |
front 186 cyclospora cayetanensis fever present | back 186 usually |
front 187 cyclospora cayetanensis blood in stool | back 187 no |
front 188 cyclospora cayetanensis distinctive features | back 188 N/A |
front 189 cyclospora cayetanensis epidemiological features | back 189 US: estimated 16,000 cases per year; internationally: endemic 27 countries, mostly tropical |
front 190 giardia lamblia mode of transmission | back 190 vehicle, fecal-oral, direct and indirect contact |
front 191 giardia lamblia virulence factors | back 191 attachment to intestines alters mucosa |
front 192 giardia lamblia culture/diagnosis | back 192 stool examination, ELISA |
front 193 giardia lamblia prevention | back 193 water hygiene, personal hygiene |
front 194 giardia lamblia treatment | back 194 tinidazole, nitazoxanide |
front 195 giardia lamblia fever present | back 195 not usually |
front 196 giardia lamblia blood in stool | back 196 no, mucus present (greasy and foul smelling) |
front 197 giardia lamblia distinctive features | back 197 frequently occurs in backpackers, campers |
front 198 giardia lamblia epidemiological features | back 198 US: estimated 1.2 million cases per year; internationally: prevalence rates from 2% to 5% in industrialized world |
front 199 entamoeba histolytica mode of transmission | back 199 vehicle, fecal-oral |
front 200 entamoeba histolytica virulence factors | back 200 lytic enzymes, induction of apoptosis, invasiveness |
front 201 entamoeba histolytica culture/diagnosis | back 201 PCR, stool examination, ELISA, serology |
front 202 entamoeba histolytica prevention | back 202 water hygiene, personal hygiene |
front 203 entamoeba histolytica treatment | back 203 metronidazole or paromomycin |
front 204 entamoeba histolytica fever present | back 204 yes |
front 205 entamoeba histolytica blood in stool | back 205 yes |
front 206 entamoeba histolytica distinctive features | back 206 N/A |
front 207 entamoeba histolytica epidemiological features | back 207 internationally: 40,000-100,000 deaths annually |
front 208 hepatitis A or E virus mode of transmission | back 208 fecal-oral, vehicle |
front 209 hepatitis A or E virus culture/diagnosis | back 209 IgM serology |
front 210 hepatitis A or E virus prevention | back 210 hepatitis a vaccine or combined; HAV/HBV vaccine |
front 211 hepatitis A or E virus treatment | back 211 HAV: hepatitis A vaccine or immune globulin; HEV: immune globulin |
front 212 hepatitis A or E virus incubation period | back 212 2-4 weeks |
front 213 hepatitis A or E virus epidemiological features | back 213 hepatitis A, US: 20,000 cases annually and 40% of adults show evidence of prior infection; internationally: 1.4 million cases per year; hepatitis E, internationally: 20 million infections per year; 60% in east and southeast asia |
front 214 hepatitis B virus mode of transmission | back 214 parenteral (blood contact), direct contact (especially sexual), vertical |
front 215 hepatitis B virus virulence factors | back 215 latency |
front 216 hepatitis B virus prevention | back 216 HMV recombinant vaccine |
front 217 hepatitis B virus culture/diagnosis | back 217 ELISA |
front 218 hepatitis B virus treatment | back 218 interferon, tenofovir, or entecavir |
front 219 hepatitis B virus incubation period | back 219 1-6 months |
front 220 hepatitis B virus epidemiological features | back 220 US: 19,000 new cases per year; 800,000 to 2.2 million have chronic infection internationally: 240 million |
front 221 hepatitis C virus mode of transmission | back 221 parenteral (blood contact), vertical |
front 222 hepatitis C virus virulence factors | back 222 core protein suppresses immune function |
front 223 hepatitis C virus culture/diagnosis | back 223 serology, also PCR |
front 224 hepatitis C virus prevention | back 224 N/A |
front 225 hepatitis C virus treatment | back 225 sofosbuvir + simeprevir |
front 226 hepatitis C virus incubation period | back 226 2-8 weeks |
front 227 hepatitis C virus epidemiological features | back 227 US: estimated 30,000 new diagnoses per year; internationally: 150 million chronically infected |
front 228 intestinal distress causative agents | back 228 enterobius vermicularis (pinworm), trichuris trichiura (whipworm), diphyllobothrium latum (fish tapeworm), hymenolepis nana and H. diminuta |
front 229 enterobius vermicularis (pinworm) disease table | back 229 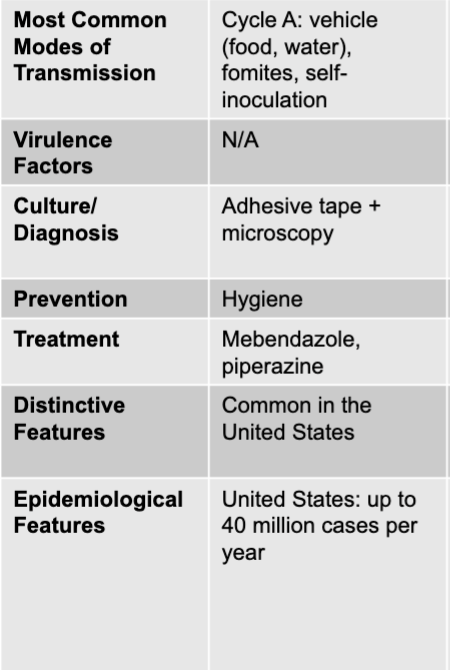 |
front 230 enterobius vermicularis (pinworm) modes of transmission | back 230 cycle A: vehicle (food, water), formites, self-inoculation |
front 231 enterobius vermicularis (pinworm) culture/diagnosis | back 231 adhesive tape + microscopy |
front 232 enterobius vermicularis (pinworm) prevention | back 232 hygiene |
front 233 enterobius vermicularis (pinworm) treatment | back 233 mebendazole, piperazine |
front 234 enterobius vermicularis (pinworm) distinctive features | back 234 common in the US |
front 235 enterobius vermicularis (pinworm) epidemiological features | back 235 US: up to 40 million cases per year |
front 236 trichuris trichiura (whipworm) disease table | back 236 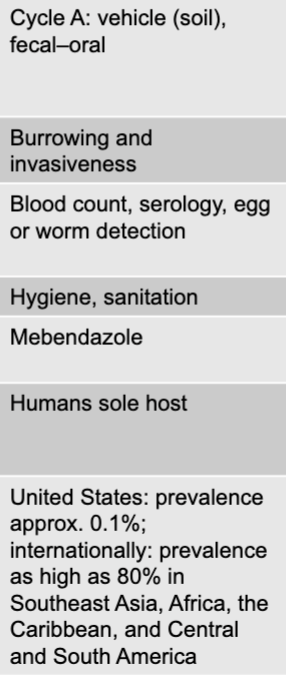 |
front 237 trichuris trichiura (whipworm) mode of transmission | back 237 cycle A: vehicle (soil), fecal-oral |
front 238 trichuris trichiura (whipworm) virulence factors | back 238 burrowing and invasiveness |
front 239 trichuris trichiura (whipworm) culture/diagnosis | back 239 blood count, serology, egg or worm detection |
front 240 trichuris trichiura (whipworm) prevention | back 240 hygiene, sanitation |
front 241 trichuris trichiura (whipworm) treatment | back 241 mebendazole |
front 242 trichuris trichiura (whipworm) distinctive features | back 242 humans sole host |
front 243 trichuris trichiura (whipworm) epidemiological features | back 243 US: prevalence approx 0.1% internationally: prevalence as high as 80% in southeast asia, africa, the caribbeam, and central and south america |
front 244 diphyllobothrium latum (fish tapeworm) disease table | back 244 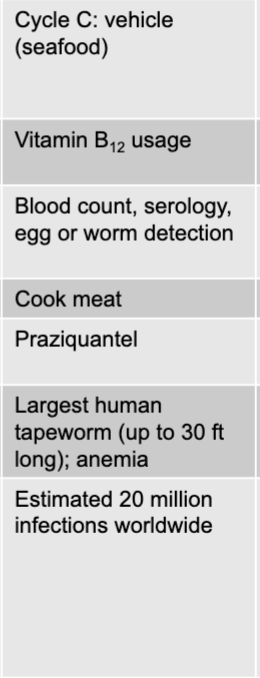 |
front 245 diphyllobothrium latum (fish tapeworm) mode of transmission | back 245 cycle C; vehicle (seafood) |
front 246 diphyllobothrium latum (fish tapeworm) virulence factors | back 246 vitamin B12 usage |
front 247 diphyllobothrium latum (fish tapeworm) culture/diagnosis | back 247 blood count, serology, egg or worm detection |
front 248 diphyllobothrium latum (fish tapeworm) prevention | back 248 cook meat |
front 249 diphyllobothrium latum (fish tapeworm) treatment | back 249 praziquantel |
front 250 diphyllobothrium latum (fish tapeworm) distinctive features | back 250 largest human tapeworm (up to 30ft long); anemia |
front 251 diphyllobothrium latum (fish tapeworm) epidemiological features | back 251 estimated 20 million infections worldwide |
front 252 hymenolepis nana and H. diminuta disease table | back 252  |
front 253 hymenolepis nana and H. diminuta mode of transmission | back 253 cycle C; vehicle (ingesting insects), fecal-oral |
front 254 hymenolepis nana and H. diminuta virulence factors | back 254 N/A |
front 255 hymenolepis nana and H. diminuta culture/diagnosis | back 255 blood count, serology, egg or worm detection |
front 256 hymenolepis nana and H. diminuta prevention | back 256 hygienic environment |
front 257 hymenolepis nana and H. diminuta treatment | back 257 praziquantel |
front 258 hymenolepis nana and H. diminuta distinctive features | back 258 most common tapeworm infection |
front 259 hymenolepis nana and H. diminuta epidemiological features | back 259 US: prevalence approximately 0.4%; internationally: the single most prevalent tapeworm infection |
front 260 intestinal distress plus migratory symptoms causative agents | back 260 toxocara species, ascaris lumbricoides (intestinal roundworm), necator americanus and ancylostoma duodenale (hookworms) |
front 261 toxocara species disease table | back 261 cycle A; dog or cat feces |
front 262 toxocara species culture/diagnosis | back 262 blood count, serology, egg or worm detection |
front 263 toxocara species prevention | back 263 hygiene |
front 264 toxocara species treatment | back 264 albendazole |
front 265 toxocara species distinctive features | back 265 can cause migration symptoms or blindness |
front 266 toxocara species epidemiological features | back 266 nearly 100% of newborn puppies in the US are infected; 14% of people in the US have been infected; considered a neglected parasitic infection |
front 267 ascaris lumbricoides (intestinal roundworm) | back 267 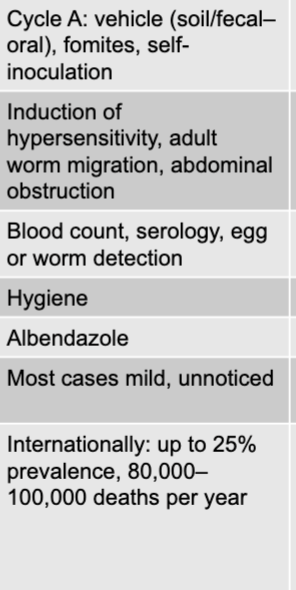 |
front 268 ascaris lumbricoides (intestinal roundworm) mode of transmission | back 268 cycle A: vehicle (soil/fecal-oral), formites, self-inoculation |
front 269 ascaris lumbricoides (intestinal roundworm) virulence factors | back 269 induction of hypersensitivity, adult worm migration, abdominal obstruction |
front 270 ascaris lumbricoides (intestinal roundworm) culture/diagnosis | back 270 blood count, serology, egg or worm detection |
front 271 ascaris lumbricoides (intestinal roundworm) prevention | back 271 hygiene |
front 272 ascaris lumbricoides (intestinal roundworm) treatment | back 272 albendazole |
front 273 ascaris lumbricoides (intestinal roundworm) distinctive features | back 273 most cases mild, unnoticed |
front 274 ascaris lumbricoides (intestinal roundworm) epidemiological features | back 274 internationally: up to 25% prevalence, 80,000-100,000 deaths per year |
front 275 necator americanus and ancylostoma duodenale (hookworms) disease table | back 275  |
front 276 necator americanus and ancylostoma duodenale (hookworms) mode of transmission | back 276 cycle B: vehicle (soil), formite |
front 277 necator americanus and ancylostoma duodenale (hookworms) virulence factors | back 277 induction of hypersensitivity, adult worm migration, abdominal obstruction |
front 278 necator americanus and ancylostoma duodenale (hookworms) culture/diagnosis | back 278 blood count, serology, egg or worm detection |
front 279 necator americanus and ancylostoma duodenale (hookworms) prevention | back 279 sanitation |
front 280 necator americanus and ancylostoma duodenale (hookworms) treatment | back 280 albendazole |
front 281 necator americanus and ancylostoma duodenale (hookworms) distinctive features | back 281 penetrates skin, serious intestinal symptoms |
front 282 necator americanus and ancylostoma duodenale (hookworms) epidemiological features | back 282 US: widespread in southeast until early 1900s; internationally: 800 million infected |
front 283 cysticercosis disease table | back 283 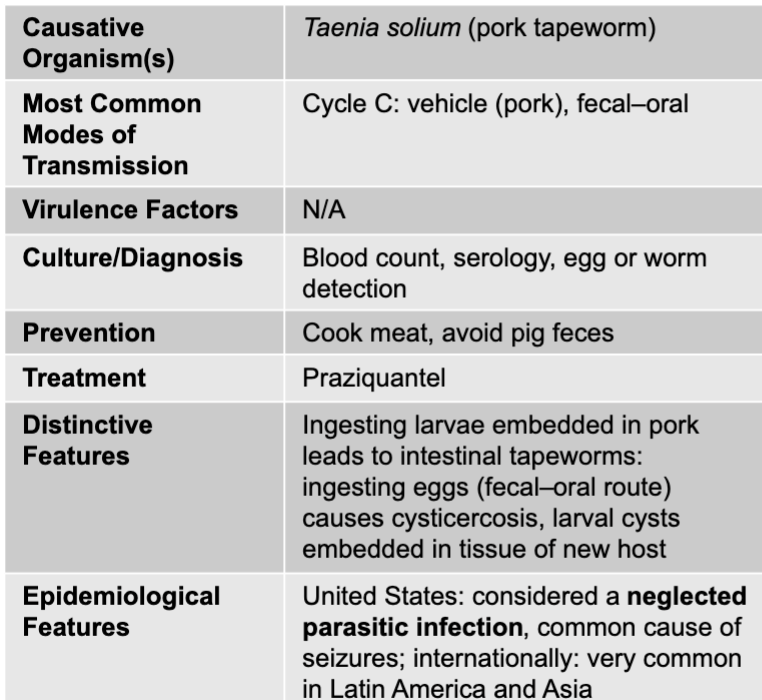 |
front 284 cysticercosis mode of transmission | back 284 cycle C: vehicle (pork), fecal-oral |
front 285 cysticercosis culture/diagnosis | back 285 blood count, serology, egg or worm detection |
front 286 cysticercosis prevention | back 286 cook meat, avoid pig feces |
front 287 cysticercosis treatment | back 287 praziquantel |
front 288 cysticercosis distinctive features | back 288 ingesting larvae embedded in pork leads to intestinal tapeworms: ingesting eggs (fecal-oral route) causes cysticercosis, larval cysts embedded in tissue of new host |
front 289 cysticercosis epidemiological features | back 289 US: considered a neglected parasitic infection, common cause of seizures; internationally: very common in latin america and asia |
front 290 liver and intestinal disease table | back 290 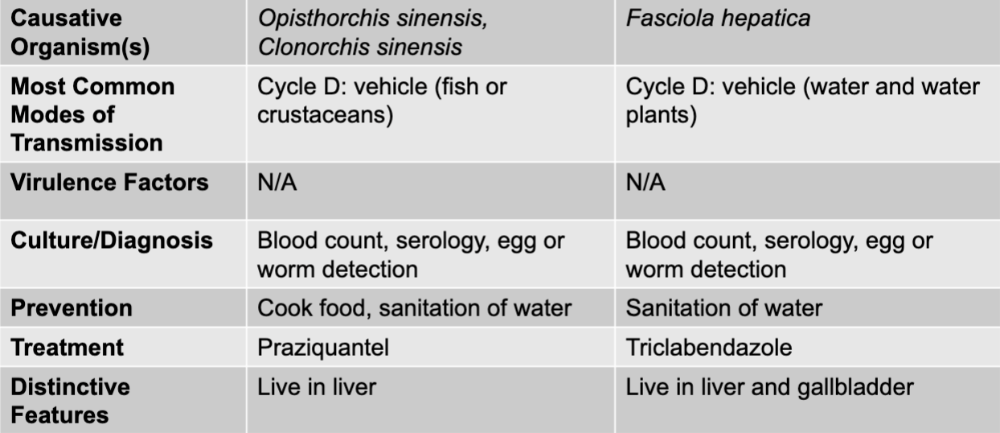 |
front 291 liver and intestinal causative agents | back 291 opisthorchis sinesis, clonorchis sinesis, and fasciola hepatica |
front 292 opisthorchis sinesis, clonorchis sinesis mode of transmission | back 292 cycle D: vehicle (fish or crustaceans) |
front 293 opisthorchis sinesis, clonorchis sinesis culture/diagnosis | back 293 blood count, serology, egg or worm detection |
front 294 opisthorchis sinesis, clonorchis sinesis prevention | back 294 cook food, sanitation of water |
front 295 opisthorchis sinesis, clonorchis sinesis treatment | back 295 praziquantel |
front 296 opisthorchis sinesis, clonorchis sinesis distinctive features | back 296 live in liver |
front 297 fasciola hepatica mode of transmission | back 297 cycle D: vehicle (water and water plants) |
front 298 fasciola hepatica culture/diagnosis | back 298 blood count, serology, egg or worm detection |
front 299 fasciola hepatica prevention | back 299 sanitation of water |
front 300 fasciola hepatica treatment | back 300 triclabendcazole |
front 301 fasciola hepatica distinctive features | back 301 live in liver and gallbladder |
front 302 muscle and neurological symptoms disease table | back 302 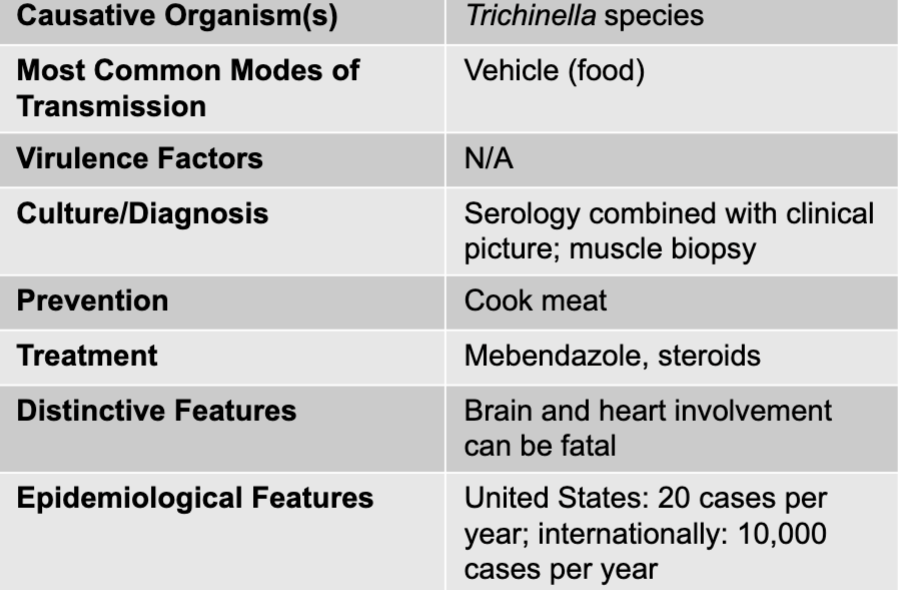 |
front 303 muscle and neurological symptoms causative agents | back 303 trichinella species |
front 304 muscle and neurological symptoms culture/diagnosis | back 304 serology combined with clinical picture; muscle biopsy |
front 305 muscle and neurological symptoms prevention | back 305 cook meat |
front 306 muscle and neurological symptoms treatment | back 306 mebendazole, steroids |
front 307 muscle and neurological symptoms distinctive features | back 307 brain and heart involvement can be fatal |
front 308 muscle and neurological symptoms epidemiological features | back 308 US: 20 cases per year; internationally: 10,000 cases per year |
front 309 schistosomiasis liver disease table | back 309 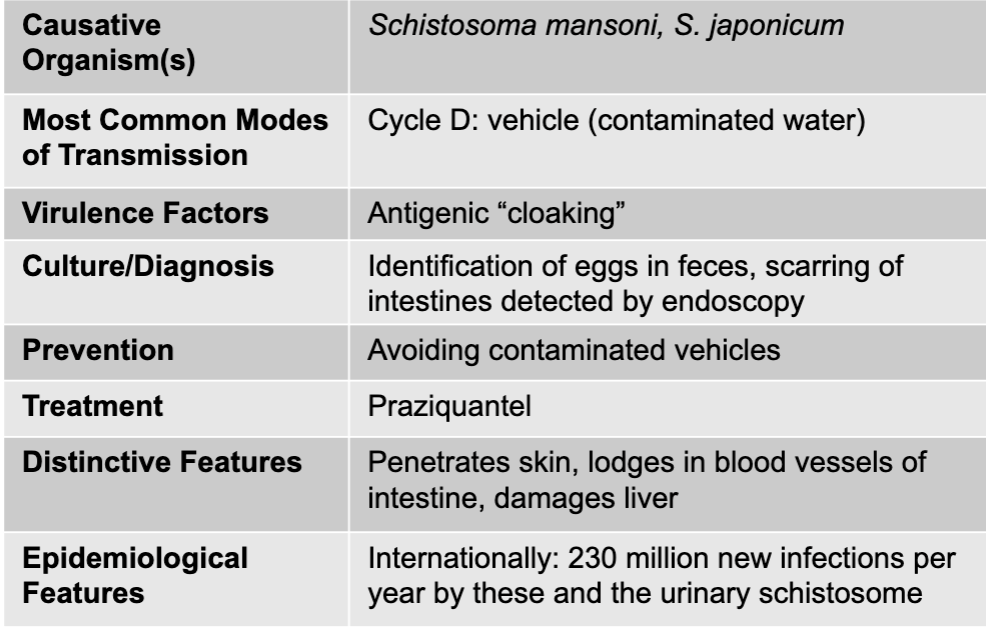 |
front 310 schistosomiasis causative agent | back 310 schistosoma mansoni, S. japonicum |
front 311 schistosomiasis mode of transmission | back 311 cycle D: vehicle (contaminated water) |
front 312 schistosomiasis virulence factors | back 312 antigenic "cloaking" |
front 313 schistosomiasis culture/diagnosis | back 313 identification of eggs in feces, scarring of intestines detected by endoscopy |
front 314 schistosomiasis prevention | back 314 avoiding contaminated vehicles |
front 315 schistosomiasis treatment | back 315 praziquantel |
front 316 schistosomiasis distinctive features | back 316 penetrates skin, lodges in blood vessels of intestine, damages liver |
front 317 schistosomiasis epidemiological features | back 317 internationally: 230 million new infections per year by these and the urinary schistosome |
front 318 gram-positive, endospore-forming bacteria | back 318 clostridioides difficile (antibiotic-associated diarrhea), bacillus cereus (food poisoning), clostridium perfringens (food poisoning) |
front 319 gram-positive bacteria | back 319 streptococcus mutans (dental caries), streptococcus sobrinus (dental caries), staphylococcus aureus (food poisoning) |
front 320 gram-negative bacteria | back 320 periodontal disease, helicobacter pylori, salmonella, shigella, escherichia coli STEC, other E.coli, campylobacter jejuni, vibrio cholera, non-cholera vibrio species |
front 321 DNA viruses | back 321 hepatitus B virus |
front 322 RNA viruses | back 322 mumps, rotavirus, norovirus, hepatitis A, E, and C |
front 323 protozoa | back 323 cryptosporidium, cyclospora cayetanesis, giardia duodenalis, enatmoeba histolytica |
front 324 helminths-nematodes | back 324 enterobius vermicularis, trichuris trichiura, toxocara species, ascaris lumbricoides, necator americanus, ancylostoma duodenale, trichinella species |
front 325 helminths-cestodes | back 325 diphyllobothrium latum, hymenolepis nana, H. diminuta, taenia solium, opisthorchis sinensis, clonorchis sinensis |
front 326 helminths-trematodes | back 326 fasciola hepatica, schistosoma mansoni, S. japonicum |