Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Micro Lab Midterm
front 1  Identify part A and its function | back 1 The stage which holds the slide in place with small clamps to be accurately viewed and allows for relocation of the slide under the objectives if desired. |
front 2  Identify part B and its function | back 2 The objective lenses which magnify the plate at 4x, 10x, 40x, and 100x. |
front 3  Identify part C and its function | back 3 The fine adjustment knob which is used for focusing the higher power objective lenses (10x, 40x, 100x) |
front 4 Define "resolution" | back 4 Ability to distinguish two separate points on a specimen |
front 5 Define "parfocal" | back 5 Refers to the ability of changing lenses without having refocusing or only refocus slightly |
front 6 Define "field of view" | back 6 Refers to the area of the plate you can see when looking through the ocular lenses (the circle we view the specimen through) |
front 7 Define "magnification" and how to calculate total magnification | back 7 The ability to enlarge an image of an object Total magnification= 10x (Ocular lens magnification) x objective lens magnification ie. ocular 10x X objective 4x = 40x total magnification |
front 8 What is the name of the microscope used in the microbiology lab? | back 8 Compound light microscope bright-field |
front 9 What is the total magnification of microscope when you are using 100x objective lens | back 9 1000x 10x (ocular) X 100x (objective) = 1000x total mag |
front 10 What is the purpose of using immersion oil? | back 10 It reduced light refraction when viewing through the 100x objective lens |
front 11 Name the structure formed in DNA caused by UV exposure | back 11 Pyrimidine dimers, or more specifically thymine dimers are formed and cause DNA damage |
front 12 What are two disadvantages of using UV for sterilization? | back 12 UV may not kill all pathogens, most effective in direct path of UV light so any sort of obstruction can render the process ineffective, harmful to other bacteria and DNA, can cause thymine dimers or other bodily damage |
front 13 What is the enzyme that can repair UV damage? | back 13 Photolyse |
front 14 At what wavelength is UV harmful to bacteria? | back 14 260 nm (tiny wavelengths) |
front 15 In the micro lab, why did we cover half of the plate while putting bacteria under the UV light? | back 15 So only half of the plate was fully exposed to the UV light. This demonstrates the effectiveness of UV sterilization on bacterial growth at the tested time intervals |
front 16 In the micro lab, which bacteria was more resistant to the UV light? Why? | back 16 Bacillus megaterium was more resistant due to the fact it produces endospores which aid in its survival and protection |
front 17 Why did we use the 3-way swab technique? Briefly explain the technique. | back 17 The 3 way swab technique was used to completely cover the side of the plate swabbed with bacteria. Since we aren't looking at the individual cells and are instead looking at colony growth patterns, we don't have to worry about crowding the plate with bacteria Technique: Using a sterile cotton swab, the bacteria is streaked across the plate from left to right, top to bottom, and diagonally to ensure an evenly covered sample for the UV test. |
front 18 What is the name of the lab instrument that sterilizes? | back 18 The autoclave |
front 19 What are the conditions for autoclaving? | back 19 15 psi 15 min 121 degrees C |
front 20 Why do we use indicators when autoclaving? | back 20 To ensure we have an accurate reading as to when everything is completely sterilized. This helps prevent sample contamination. |
front 21 Describe 3 important steps in preparing a smear for staining. | back 21 Proper inoculation of the plate depending on the sample used (broth, plate, slant), complete airdrying, and thoroughly heat-fixing |
front 22 Describe 4 important steps in the preparation of a smear from agar. | back 22 Adding a singular drop of water, proper + sterile plate inoculation, complete airdrying, and thoroughly heat-fixing. |
front 23 What happens if you grab too many bacteria cells for a gram stain? | back 23 The plate will be overcrowded when looking through the microscope and you will not be able to accurately see the individual cells morphology |
front 24 What will happen if you don't heat-fix before beginning the staining process? | back 24 During the staining process the bacteria will be washed off the plate |
front 25 What will happen if you don't completely airdry before heat fixing the plate during a smear prep? | back 25 When heat-fixing, the residual moisture will steam and kill/disfigure the bacterial cells |
front 26  Classify organism in tube A (generalized by reaction to oxygen) | back 26 Facultative anaerobe |
front 27  Classify organism in tube B (generalized by reaction to oxygen) | back 27 Microaerophile |
front 28  Classify organism in tube C (generalized by reaction to oxygen) | back 28 microaerophile |
front 29 What is the name of the media used in the oxygen tolerance test | back 29 fluid thioglycolate medium |
front 30 What does "MSA" stand for? Give the name of the inhibitor and indicator in MSA. | back 30 MSA= Mannitol salt agar Indicator: Phenol Red Inhibitor: salt 7.5% |
front 31 What is the name of the indicator used in the 2042 oxygen tolerance test? | back 31 Resazurin |
front 32 What type of bacteria grows in MSA? | back 32 Staphylococci |
front 33 What is the inhibitor and indicator in MacConkey media? | back 33 Inhibitor: bile salt indicator: neutral red |
front 34 What bacteria does MacConkey promote the growth of? | back 34 Gram negative and enteric bacteria |
front 35 What is hemolysis? Define alpha, beta, and gamma hemolysis. | back 35 Hemolysis: ability to break down blood cells alpha: partial hemolysis beta: full hemolysis gamma: no hemolysis |
front 36 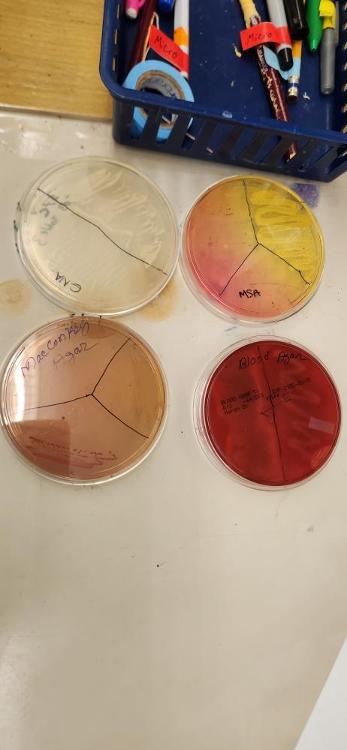 What type of hemolysis is shown on plate E? | back 36 Beta hemolysis |
front 37 What does Columbia "CNA" stand for? What is the main inhibitor in Columbia CNA media? | back 37 Columbia CNA= colistin nalidixic acid agar Inhibitors: Colistin/nalidixic acid |
front 38 What bacteria grows in Columbia CNA agar? | back 38 Gram positive bacteria |
front 39  Does this tube represent a positive or negative test? What is the name of the inverted tube in the glucose/lactose test tube? What does this tube test for? | back 39 This test is positive shown by the bright yellow color (formerly bright red) which means there was carbohydrate fermentation Durham tube If the tube appears clear and/or has a bubble in it, the test is positive for gas production |
front 40 What is the name for the technique used to streak a plate to isolate colonies? | back 40 4 quadrant streak method |
front 41 Define a pure vs a mixed culture. | back 41 A pure culture contains only one bacteria while a mixed culture contains multiple bacteria |
front 42 What type of media would you use to determine if a culture is mixed or pure? | back 42 Differential media which allows for multiple different bacteria to grow at once |
front 43 Which did we use for our lab experiments: pure or mixed cultures? | back 43 We used both to see the differentiation in a mixed and pure culture |
front 44 What happens if you omit flaming the loop before inoculation of the media? | back 44 Flaming is a technique used for sterilization so if omitted could lead to contaminated cultures and inaccurate results. |
front 45 Do we take culture for inoculation for each quadrant in the 4-quadrant streaking method? Why or why not? What is the proper way of streaking the 4 quadrants? | back 45 No, it could lead to different DNA cultures rather than a pure, isolated colony. Instead, you flame the loop in between each quadrant and continue the original line of inoculation through each quadrant. |
front 46 What position are plates incubated in the incubator? | back 46 Upside-down to prevent lids falling off or plates getting mixed up |
front 47 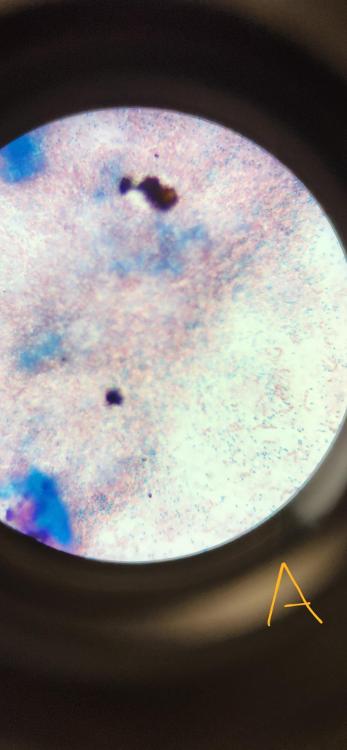 What staining technique was used in the provided picture? | back 47 Endospore stain (Schaeffer-Futton method) |
front 48 What are the dyes and decolorizer used for a gram stain (in order) | back 48 malachite green (primary stain) DI water (decolorizer) safranin (counterstain) |
front 49 In the endospore stain done in lab, what were the green-stained specimen and what were the pink-stained specimen? | back 49 The green was the malachite green stained endospores The pink was safranin stained gram negative rods |
front 50  What bacteria was used in this stain? | back 50 Clostridium sporogenes |
front 51  What staining technique was used in this picture? | back 51 gram stain |
front 52  What bacteria was featured in this gram stain? | back 52 Bacillus megaterium |
front 53 What are the dyes and decolorizer for a gram stain? | back 53 Crystal violet (primary) iodine (mordant) ethyl alcohol (decolorizer) safranin (counter stain) |
front 54 What is the purpose of each component of a gram stain? | back 54 Primary stain is used to color the bacteria walls the mordant is used to bind the primary stain to the walls the decolorizer is used to remove the dye from everything but the dyed bacteria the counterstain is to differentiate any gram-negative bacteria that is unable to be stained from the primary stain |
front 55  What is the name of the media present? | back 55 Agar plate; solid media |
front 56  What is the name of the media present? | back 56 nutrient broth; liquid media |
front 57  What is the name of the media present? | back 57 Agar slant; solid media |
front 58 Why do we use agar instead of gelatin? | back 58 Gelatin melts at room temperature and can sometimes be eaten away at by the bacteria cultures; agar cannot |
front 59 Define "osmosis". | back 59 The diffusion of water across a semi-permeable membrane |