Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Chapter 2 - Acids and Bases
front 1  Label the Bronsted Lowry acid, base, conjugate acid and conjugate base. | back 1  |
front 2 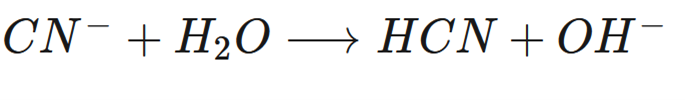 Label the Bronsted Lowry acid, base, conjugate acid and conjugate base. | back 2 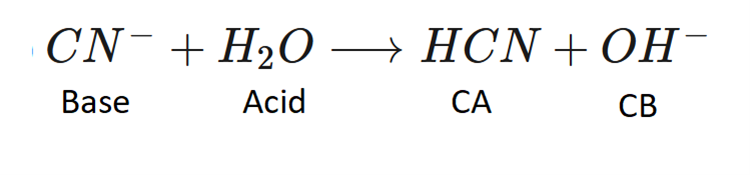 |
front 3 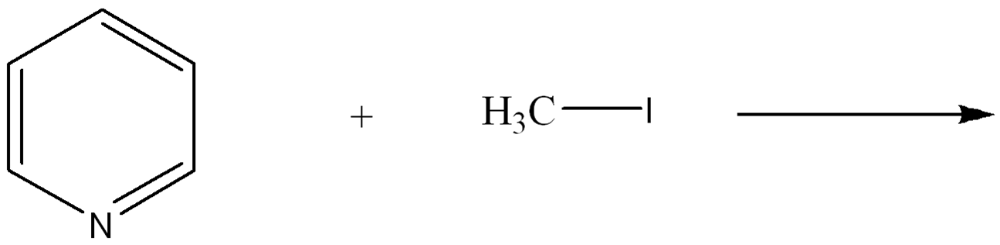 Use curved arrow notation to show the movement of electrons in the Lewis Acid-Base Reaction | back 3 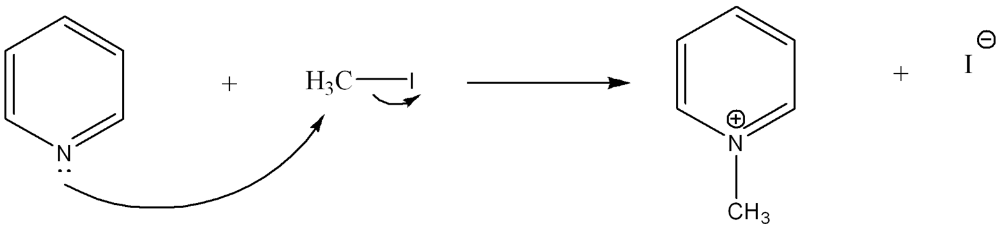 |
front 4 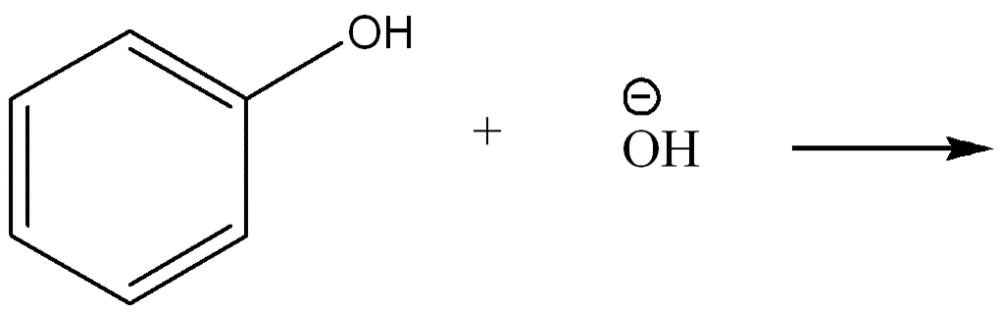 Use curved arrow notation to show the movement of electrons in the Bronsted Lowry Acid-Base Reaction | back 4 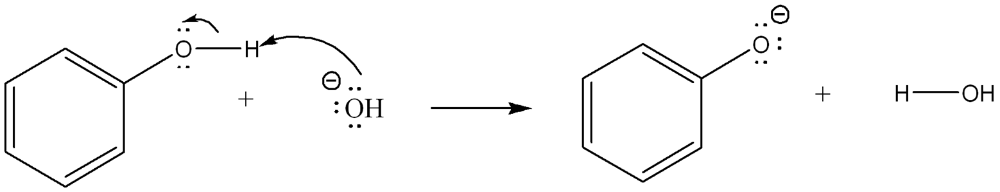 |
front 5 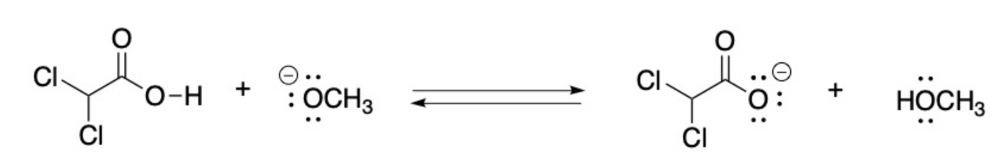 Label the Bronsted Lowry acid, base, conjugate acid and conjugate base. Then determine in which direction equilibrium is favored. | back 5 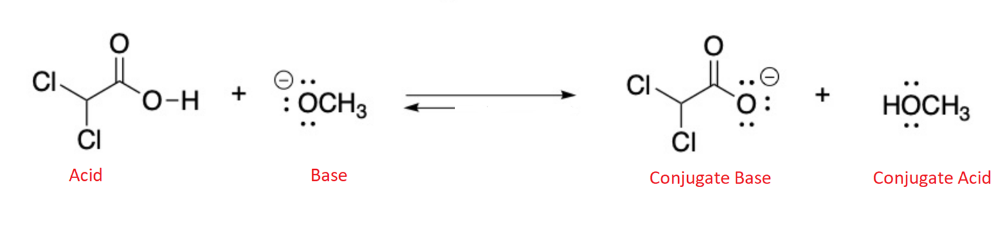 |
front 6 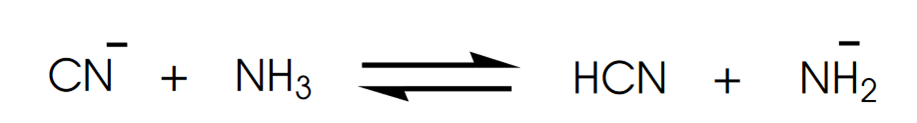 Determine in which direction equilibrium is favored. | back 6 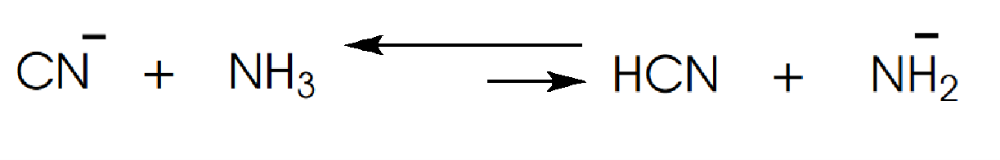 |
front 7 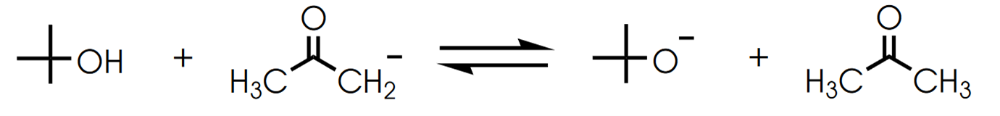 Determine in which direction equilibrium is favored. | back 7 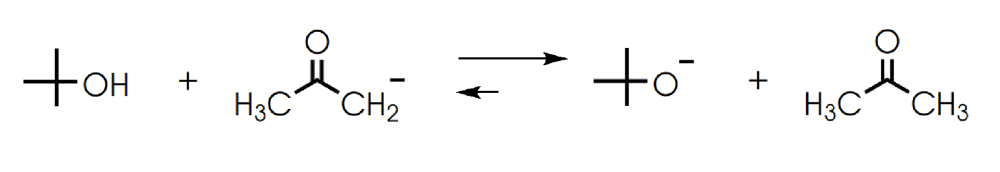 |
front 8 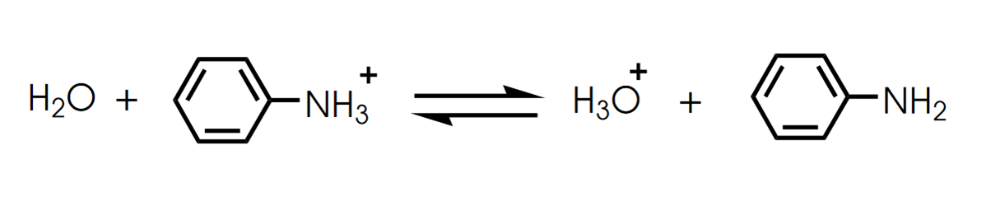 Determine in which direction equilibrium is favored. | back 8 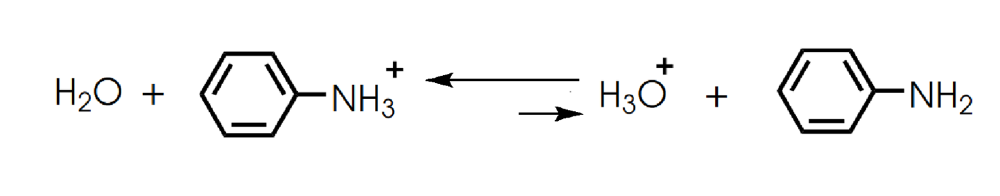 |
front 9 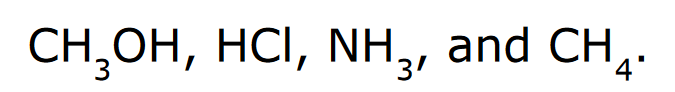 Rank the following in order of increasing acidity | back 9 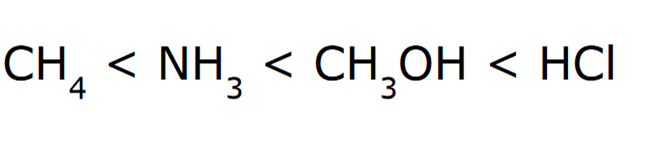 *Elemental Effect Electronegativity when comparing CH4, NH3, and CH3OH Polarizability when comparing CH3OH and HCl |
front 10 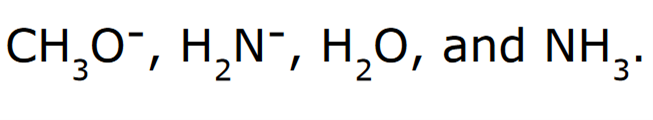 Rank the following in order of increasing basicity | back 10 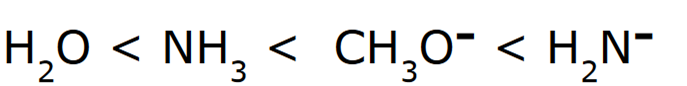 Negatively charged ions are stronger bases compared to their neutral counterparts When comparing H2O and NH3, H2O is a stronger acid, because the conjugate base (OH-) is more stable than (NH2-) because oxygen is more electronegative than nitrogen. Since OH- is the more stable conjugate base, it is a stronger acid, and thereby the weakest base. |
front 11  Rank these compounds in order of increasing acidity | back 11 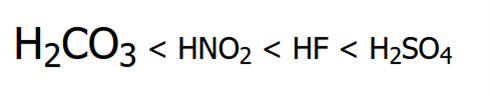 |
front 12 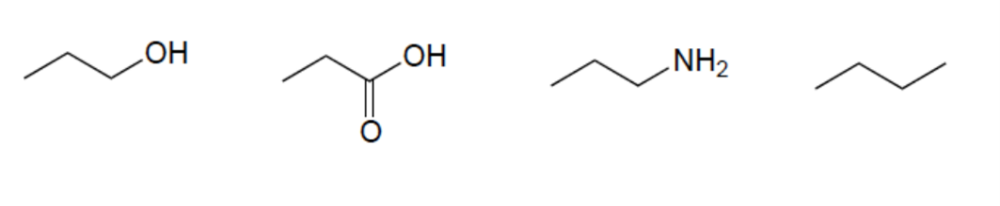 Rank these compounds in order of increasing acidity | back 12 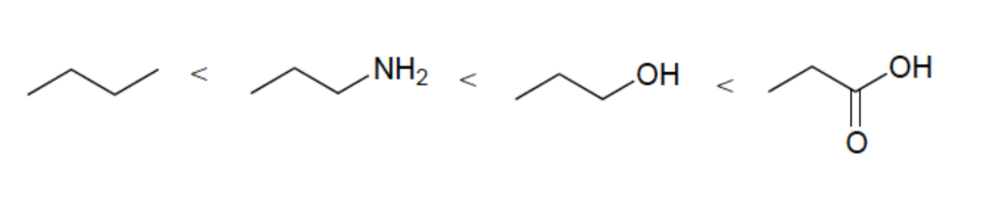 Elemental Effect (CH3 vs NH2) Elemental Effect (NH2 vs OH) Resonance Effect (OH vs. CO2H) |
front 13 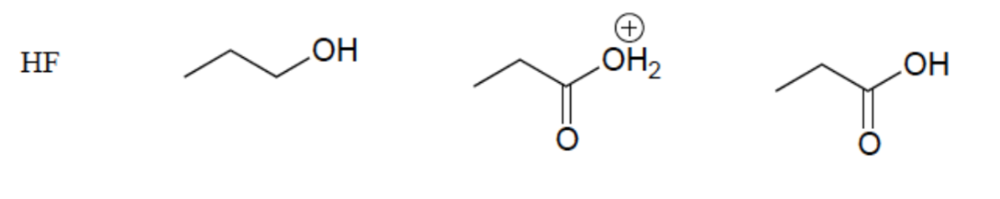 Rank these compounds in order of increasing acidity | back 13 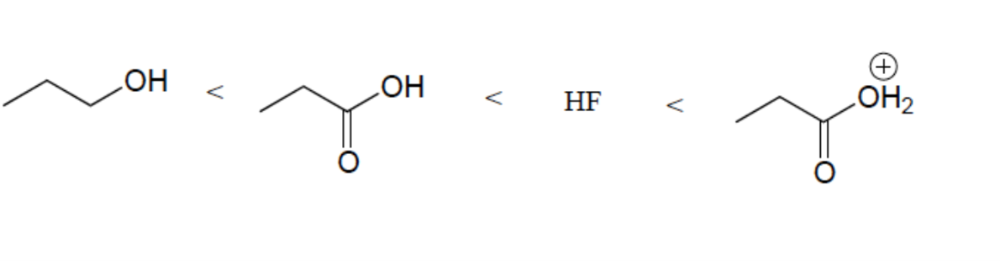 Resonance Effect (OH vs CO2H) Elemental Effect (CO2H vs HF) Positively charged ions are stronger acids than neutral counterparts |
front 14 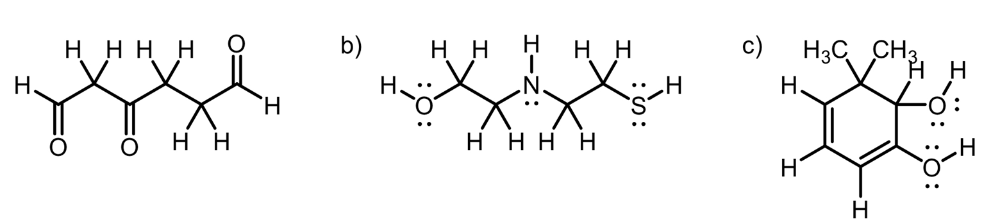 Circle the most acidic proton on each compound | back 14 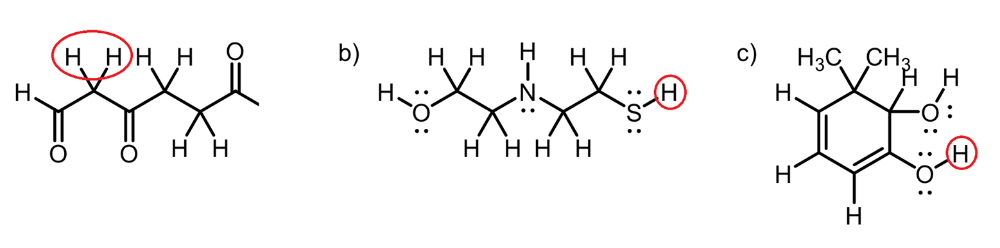 A. Inductive Effect B. Elemental Effect (Eliminates C-H and N-H), (Sulfur is more polarizable than Oxygen) C. Elemental Effect (Eliminates C-H), Resonance Effect (Eliminates O-H group) |
front 15 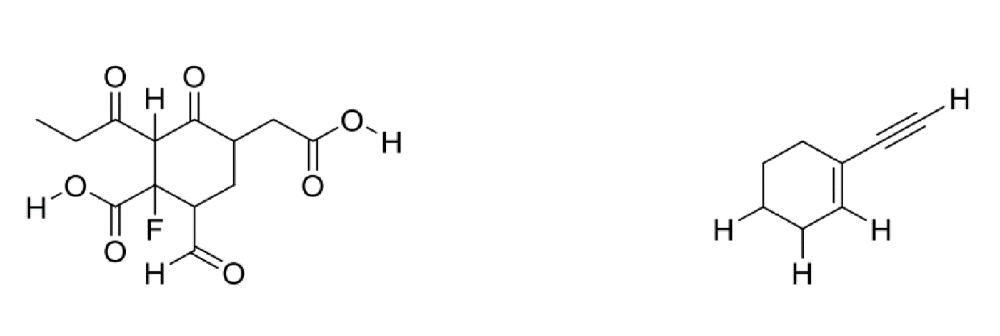 Circle the most acidic proton on each compound | back 15 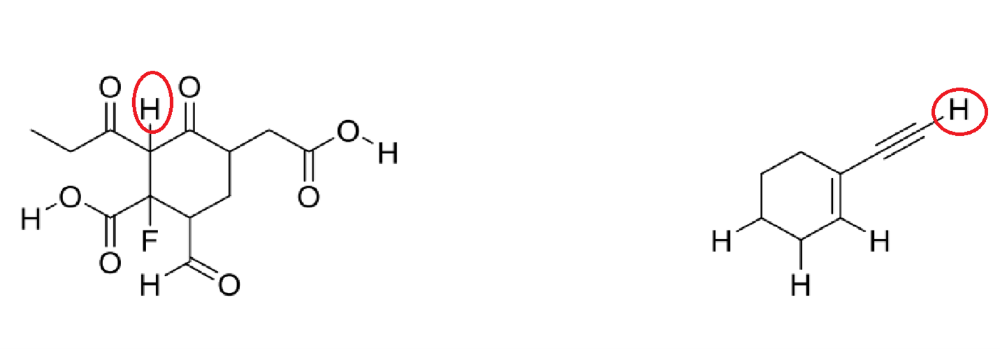 A. Elemental Effect (Fluorine electronegativity) B. Hybridization Effect (increase percent s character, closer to the nucleus, more stable) |
front 16  Draw the products for the Lewis Acid and Base Reaction. Identify the electrophile and nucleophile | back 16 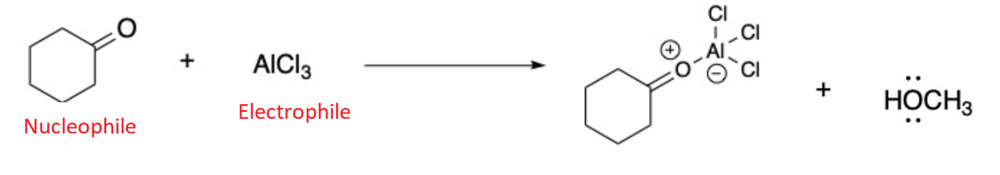 |
front 17 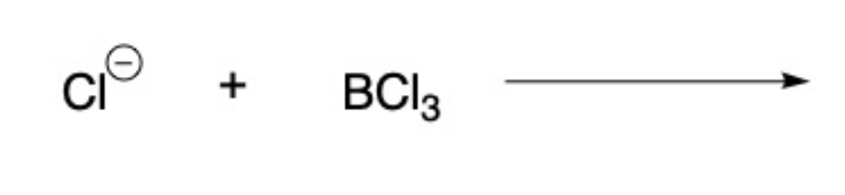 Draw the products for the Lewis Acid and Base Reaction. Identify the electrophile and nucleophile | back 17  |