Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Central Science: Chapter 2
front 1 A molecule of water contains hydrogen and oxygen in a 1:8 ratio by
mass. This is a statement of ________. | back 1 B |
front 2 Which one of the following is not one of the postulates
of Dalton's atomic theory? | back 2 B |
front 3 Consider the following selected postulates of Dalton's atomic
theory: Which of the postulates is(are) no longer considered valid? | back 3 C |
front 4 Which pair of substances could be used to illustrate the law of
multiple proportions? | back 4 B |
front 5 Which statement below correctly describes the responses of alpha,
beta, and gamma radiation to an electric field? | back 5 D |
front 6 Which one of the following is not true concerning cathode
rays? | back 6 E |
front 7 The charge on an electron was determined in the ________. | back 7 C |
front 8 ________-rays consist of fast-moving electrons. | back 8 B |
front 9 The gold foil experiment performed in Rutherford's lab
________. | back 9 B |
front 10 In the Rutherford nuclear-atom model, ________. | back 10 A |
front 11 Cathode rays are ________. | back 11 C |
front 12 Cathode rays are deflected away from a negatively charged plate
because ________. | back 12 D |
front 13 Cathode rays are deflected away from a negatively charged plate
because ________. | back 13 B |
front 14 Of the three types of radioactivity characterized by Rutherford,
which is/are electrically charged? | back 14 B |
front 15 Of the three types of radioactivity characterized by Rutherford,
which is/are not electrically charged? | back 15 C |
front 16 Of the three types of radioactivity characterized by Rutherford,
which are particles? | back 16 E |
front 17 Of the three types of radioactivity characterized by Rutherford,
which type does not become deflected by a electric field? | back 17 D |
front 18 Of the following, the smallest and lightest subatomic particle is the
________. | back 18 C |
front 19 All atoms of a given element have the same ________. | back 19 B |
front 20 Which atom has the smallest number of neutrons? | back 20 B |
front 21 Which of the following atoms has the smallest number of
neutrons? | back 21 C |
front 22 There are ________ electrons, ________ protons, and ________ neutrons
in an atom of 132 54Xe. | back 22 D |
front 23 An atom of the most common isotope of gold, 197Au, has
________ protons, ________ neutrons, and ________ electrons. | back 23 E |
front 24 Which combination of protons, neutrons, and electrons is correct for
the isotope of copper, 63
29Cu? | back 24 A |
front 25 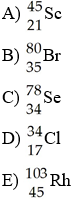 Which isotope has 45 neutrons? | back 25 B |
front 26 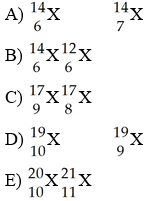 Which pair of atoms constitutes a pair of isotopes of the same element? | back 26 B |
front 27 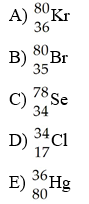 Which isotope has 36 electrons in an atom? | back 27 A |
front 28 Isotopes are atoms that have the same ________ but differing
________. | back 28 C |
front 29 The nucleus of an atom does not contain ________. | back 29 E |
front 30 The subatomic particles located in the nucleus with no overall
charges are ________. | back 30 C |
front 31 Different isotopes of a particular element contain the same number of
________. | back 31 A |
front 32 Different isotopes of a particular element contain different numbers
of ________. | back 32 B |
front 33 In the symbol shown below, x = ________. | back 33 D |
front 34 In the symbol below, X = ________. | back 34 B |
front 35 In the symbol below, x = ________. | back 35 E |
front 36 In the symbol below, x is ________. | back 36 C |
front 37 Which one of the following basic forces is so small that it has no
chemical significance? | back 37 D |
front 38 Gravitational forces act between objects in proportion to their
________. | back 38 B |
front 39 The average atomic mass of silver is 107.8682 amu. The fractional
abundance of the lighter of the two isotopes is ________. | back 39 C |
front 40 The atomic mass unit is presently based on assigning an exact
integral mass (in amu) to an isotope of ________. | back 40 D |
front 41 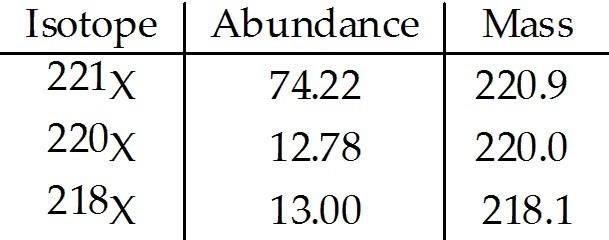 The element X has three naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is ________ amu. A) 219.7 | back 41 B |
front 42  Element X has three naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is ________ amu. A) 41.54 | back 42 A |
front 43 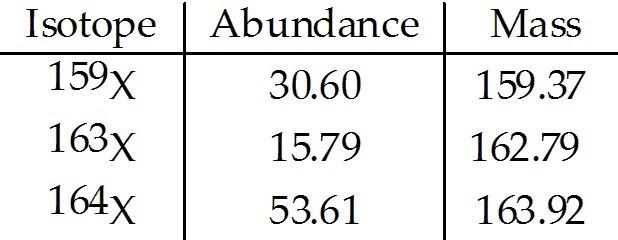 The element X has three naturally occurring isotopes. The isotopic masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is ________ amu. A) 161.75 | back 43 C |
front 44  The element X has three naturally occurring isotopes. The isotopic masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is ________ amu. A) 33.33 | back 44 C |
front 45  The element X has two naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is ________ amu. A) 30.20 | back 45 B |
front 46 The average atomic weight of copper, which has two naturally
occurring isotopes, is 63.5. One of the isotopes has an atomic weight
of 62.9 amu and constitutes 69.1% of the copper isotopes. The other
isotope has an abundance of 30.9%. The atomic weight (amu) of the
second isotope is ________ amu. | back 46 D |
front 47 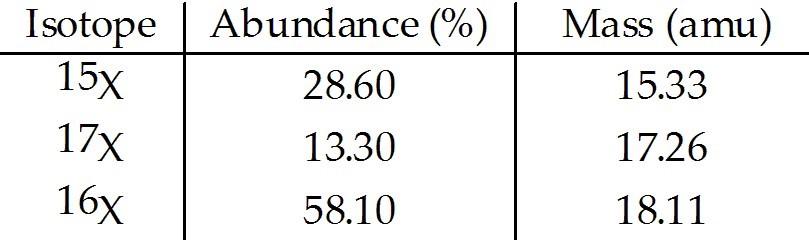 The element X has three naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is ________ amu. A) 17.20 | back 47 A |
front 48 Vanadium has two naturally occurring isotopes, 50V with an
atomic mass of 49.9472 amu and 51V with an atomic mass of
50.9440. The atomic weight of vanadium is 50.9415. The percent
abundances of the vanadium isotopes are ________% 50V and
________% 51V. | back 48 A |
front 49 An unknown element is found to have three naturally occurring
isotopes with atomic masses of 35.9675 (0.337%), 37.9627 (0.063%), and
39.9624 (99.600%). Which of the following is the unknown
element? | back 49 A |
front 50 In the periodic table, the elements are arranged in ________.
| back 50 B |
front 51 Elements ________ exhibit similar physical and chemical
properties. | back 51 E |
front 52 Which pair of elements would you expect to exhibit the greatest
similarity in their physical and chemical properties? | back 52 C |
front 53 Which pair of elements would you expect to exhibit the greatest
similarity in their physical and chemical properties? | back 53 A |
front 54 Which pair of elements would you expect to exhibit the greatest
similarity in their physical and chemical properties? | back 54 C |
front 55 The elements in groups 1A, 6A, and 7A are called ________,
respectively. | back 55 B |
front 56 Which pair of elements below should be the most similar in chemical
properties? | back 56 C |
front 57 An element in the upper right corner of the periodic table
________. | back 57 D |
front 58 An element that appears in the lower left corner of the periodic
table is ________. | back 58 B |
front 59 Elements in the same group of the periodic table typically have
________. | back 59 E |
front 60 Which one of the following molecular formulas is also an empirical
formula? | back 60 B |
front 61 Which compounds do not have the same empirical formula? | back 61 B |
front 62 Of the choices below, which one is not an ionic
compound? | back 62 A |
front 63 Which type of formula provides the most information about a
compound? | back 63 D |
front 64 A molecular formula always indicates ________. | back 64 A |
front 65 An empirical formula always indicates ________. | back 65 C |
front 66 The molecular formula of a compound is always ________ the empirical
formula. | back 66 C |
front 67 Formulas that show how atoms are attached in a molecule are called
________. | back 67 E |
front 68 Of the following, ________ contains the greatest number of
electrons. | back 68 D |
front 69 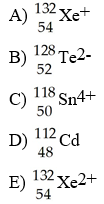 Which species has 54 electrons? | back 69 B |
front 70 Which species has 16 protons? | back 70 B |
front 71 Which species has 18 electrons? | back 71 B |
front 72 Which of the following species contains 18 electrons? | back 72 B |
front 73 Which of the following species is an isotope of 79Br? | back 73 D |
front 74 Which one of the following species has as many electrons as it has
neutrons? | back 74 D |
front 75 There are ________ protons, ________ neutrons, and ________ electrons
in 131I-. | back 75 C |
front 76 There are ________ protons, ________ neutrons, and ________ electrons
in 238U+5. | back 76 B |
front 77 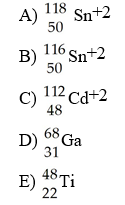 Which species contains 68 neutrons? | back 77 A |
front 78 Which of the following compounds would you expect to be
ionic? | back 78 C |
front 79 Which pair of elements is most apt to form an ionic compound with
each other? | back 79 A |
front 80 Which pair of elements is most apt to form a molecular compound with
each other? | back 80 C |
front 81 Which species below is the nitride ion? | back 81 E |
front 82 Barium reacts with a polyatomic ion to form a compound with the
general formula Ba3(X)2. What would be the most likely formula for the
compound formed between sodium and the polyatomic ion X? | back 82 D |
front 83 Aluminum reacts with a certain nonmetallic element to form a compound
with the general formula Al2X3. Element X must be from Group ________
of the Periodic Table of Elements. | back 83 D |
front 84 The formula for a salt is XBr. The X-ion in this salt has 46
electrons. The metal X is ________. | back 84 A |
front 85 Which formula/name pair is incorrect? | back 85 D |
front 86 Which formula/name pair is incorrect? | back 86 E |
front 87 Which one of the following is the formula of hydrochloric
acid? | back 87 D |
front 88 The suffix -ide is used primarily ________. | back 88 A |
front 89 Which one of the following compounds is chromium(III) oxide? | back 89 A |
front 90 Which one of the following compounds is copper(I) chloride? | back 90 A |
front 91 The correct name for MgF2 is ________. | back 91 E |
front 92 The correct name for NaHCO3 is ________. | back 92 D |
front 93 A correct name for Fe(NO3)2 is ________. | back 93 C |
front 94 The correct name for HNO2 is ________. | back 94 A |
front 95 The proper formula for the hydronium ion is ________. | back 95 D |
front 96 The charge on the ________ ion is -3. | back 96 E |
front 97 Which one of the following polyatomic ions has the same charge as the
hydroxide ion? | back 97 C |
front 98 Which element forms an ion with the same charge as the ammonium
ion? | back 98 A |
front 99 The formula for the compound formed between aluminum ions and
phosphate ions is ________. | back 99 B |
front 100 Which metal does not
form cations of differing charges? | back 100 A |
front 101 Which metal forms cations of differing charges? | back 101 E |
front 102 The correct name for Ni(CN)2 is ________. | back 102 D |
front 103 What is the molecular formula for 1-propanol? | back 103 C |
front 104 Methane and ethane are both made up of carbon and hydrogen. In
methane, there are 12.0 g of carbon for every 4.00 g of hydrogen, a
ratio of 3:1 by mass. In ethane, there are 24.0 g of carbon for every
6.00 g of hydrogen, a ratio of 4:1 by mass. This is an illustration of
the law of ________. | back 104 B |
front 105 ________ and ________ reside in the atomic nucleus. | back 105 C |
front 106 520 pm is the same as ________ Å. | back 106 D |
front 107 The atomic number indicates ________. | back 107 C |
front 108 The nucleus of an atom contains ________. | back 108 C |
front 109 In the periodic table, the elements touching the steplike line are
known as ________. | back 109 C |
front 110 Which group in the periodic table contains only nonmetals? | back 110 E |
front 111 Horizontal rows of the periodic table are known as ________. | back 111 A |
front 112 Vertical columns of the periodic table are known as ________. | back 112 D |
front 113 Elements in Group 1A are known as the ________. | back 113 C |
front 114 Elements in Group 2A are known as the ________. | back 114 A |
front 115 Elements in Group 6A are known as the ________. | back 115 B |
front 116 Elements in Group 7A are known as the ________. | back 116 D |
front 117 Elements in Group 8A are known as the ________. | back 117 E |
front 118 Potassium is a ________ and chlorine is a ________. | back 118 A |
front 119 Lithium is a ________ and magnesium is a ________. | back 119 C |
front 120 Oxygen is a ________ and nitrogen is a ________. | back 120 D |
front 121 Calcium is a ________ and silver is a ________. | back 121 B |
front 122 ________ are found uncombined, as monatomic species in
nature. | back 122 A |
front 123 When a metal and a nonmetal react, the ________ tends to lose
electrons and the ________ tends to gain electrons. | back 123 C |
front 124 The empirical formula of a compound with molecules containing 12
carbon atoms, 14 hydrogen atoms, and 6 oxygen atoms is
________. | back 124 D |
front 125 ________ only form ions with a 2+ charge. | back 125 A |
front 126 What is the formula of the compound formed between strontium ions and
nitrogen ions? | back 126 B |
front 127 Magnesium reacts with a certain element to form a compound with the
general formula MgX. What would the most likely formula be for the
compound formed between potassium and element X? | back 127 A |
front 128 The charge on the manganese in the salt MnF3 is ________. | back 128 E |
front 129 Aluminum reacts with a certain nonmetallic element to form a compound
with the general formula AlX. Element X is a diatomic gas at room
temperature. Element X must be ________. | back 129 D |
front 130 Sodium forms an ion with a charge of ________. | back 130 A |
front 131 Potassium forms an ion with a charge of ________. | back 131 C |
front 132 Calcium forms an ion with a charge of ________. | back 132 D |
front 133 Barium forms an ion with a charge of ________. | back 133 E |
front 134 Aluminum forms an ion with a charge of ________. | back 134 D |
front 135 Fluorine forms an ion with a charge of ________. | back 135 A |
front 136 Iodine forms an ion with a charge of ________. | back 136 E |
front 137 Oxygen forms an ion with a charge of ________. | back 137 A |
front 138 Sulfur forms an ion with a charge of ________. | back 138 B |
front 139 Predict the empirical formula of the ionic compound that forms from
sodium and fluorine. | back 139 A |
front 140 Predict the empirical formula of the ionic compound that forms from
magnesium and fluorine. | back 140 E |
front 141 Predict the empirical formula of the ionic compound that forms from
magnesium and oxygen. | back 141 B |
front 142 Predict the empirical formula of the ionic compound that forms from
aluminum and oxygen. | back 142 C |
front 143 The correct name for K2S is ________. | back 143 D |
front 144 The correct name for Al2O3 is ________. | back 144 A |
front 145 The correct name for CaH2 is ________. | back 145 E |
front 146 The correct name for SO is ________. | back 146 B |
front 147 The correct name for CCl4 is ________. | back 147 D |
front 148 The correct name for N2O5 is ________. | back 148 C |
front 149 The correct name for H2CO3 is ________. | back 149 C |
front 150 The correct name for H2SO3 is ________. | back 150 B |
front 151 The correct name for H2SO4 is ________. | back 151 A |
front 152 The correct name for HNO3 is ________. | back 152 B |
front 153 The correct name for HClO3 is ________. | back 153 C |
front 154 The correct name for HClO is ________. | back 154 E |
front 155 The correct name for HBrO4 is ________. | back 155 B |
front 156 The correct name for HBrO is ________. | back 156 E |
front 157 The correct name for HBrO2 is ________. | back 157 D |
front 158 The correct name for HClO2 is ________. | back 158 E |
front 159 The correct name of the compound Na3N is ________. | back 159 A |
front 160 The formula of bromic acid is ________. | back 160 D |
front 161 The correct formula for molybdenum (IV) hypochlorite is
________. | back 161 B |
front 162 The name of PCl3 is ________. | back 162 B |
front 163 The ions Ca2+ and PO43- form a salt with the
formula ________. | back 163 E |
front 164 The correct formula of iron (III) bromide is ________. | back 164 B |
front 165 Magnesium and sulfur form an ionic compound with the formula
________. | back 165 A |
front 166 The formula of ammonium carbonate is ________. | back 166 A |
front 167 The formula of the chromate ion is ________. | back 167 A |
front 168 The formula of the carbonate ion is ________. | back 168 B |
front 169 The correct name for Mg(ClO3)2 is ________. | back 169 A |
front 170 What is the correct formula for ammonium sulfide? | back 170 C |
front 171 When calcium reacts with sulfur the compound formed is
________. | back 171 C |
front 172 Chromium and chlorine form an ionic compound whose formula is CrCl3.
The name of this compound is ________. | back 172 B |
front 173 Iron and chlorine form an ionic compound whose formula is FeCl3. The
name of this compound is ________. | back 173 B |
front 174 Copper and chlorine form an ionic compound whose formula is CuCl2.
The name of this compound is ________. | back 174 E |
front 175 The name of the binary compound N2O4 is ________. | back 175 D |
front 176 The formula for zinc phosphate is Zn3(PO4)2. What is the formula for
cadmium arsenate? | back 176 B |
front 177 The formula for aluminum hydroxide is ________. | back 177 D |
front 178 The name of the ionic compound V2O3 is ________. | back 178 A |
front 179 The name of the ionic compound NH4CN is ________. | back 179 C |
front 180 The name of the ionic compound (NH4)3PO4 is ________. | back 180 A |
front 181 What is the formula for perchloric acid? | back 181 C |
front 182 The correct name for HIO2 is ________. | back 182 D |
front 183 What is the molecular formula for propane? | back 183 C |
front 184 What is the molecular formula for butane? | back 184 E |
front 185 What are the primary atoms found in alkanes? | back 185 E |
front 186 What is the correct name for the following alkane, C5H12? | back 186 D |
front 187 How many carbon and hydrogen atoms are found in decane? | back 187 A |
front 188 What is the molecular formula for heptane? | back 188 D |
front 189 What is the molecular formula for 1-hexanol? | back 189 B |
front 190 A certain mass of carbon reacts with 128 g of oxygen to form carbon
monoxide. ________ grams of oxygen would react with that same mass of
carbon to form carbon dioxide, according to the law of multiple
proportions. | back 190 E |
front 191 An atom of 13C contains ________ protons. | back 191 A |
front 192 Of the following, the subatomic particle with the smallest mass is
the ________. | back 192 C |
front 193 An atom of 118Xe contains ________ neutrons. | back 193 C |
front 194 There are ________ protons, ________ electrons, and ________ neutrons
in an atom of Xe. | back 194 D |
front 195 An atom of 14C contains ________ electrons. | back 195 E |
front 196 87 pm is the same as ________ Angstroms. | back 196 D |
front 197 200 pm is the same as ________ Å. | back 197 D |
front 198 In the symbol below, X = ________. | back 198 B |
front 199 In the symbol below, x = ________. | back 199 E |
front 200 The mass number of an atom of 14C is ________. | back 200 D |
front 201 Which atom has the largest number of neutrons? | back 201 D |
front 202 How many neutrons are there in one atom of 184W? | back 202 D |
front 203 How many protons are there in one atom of 71Ga? | back 203 D |
front 204 How many electrons are there in one atom of 71Ga? | back 204 D |
front 205  Which pair of atoms constitutes a pair of isotopes of the same element? | back 205 B |
front 206 The atomic number of an atom of 80Br is ________. | back 206 B |
front 207 How many total electrons are in the Li+ ion? | back 207 A |
front 208 How many total electrons are in the O2- ion? | back 208 A |
front 209 If a iron atom loses 2 electrons to make an ion, what is the charge
on that ion? | back 209 A |
front 210 If an atom gains 3 electrons to make an ion, what is the charge on
that ion? | back 210 E |
front 211 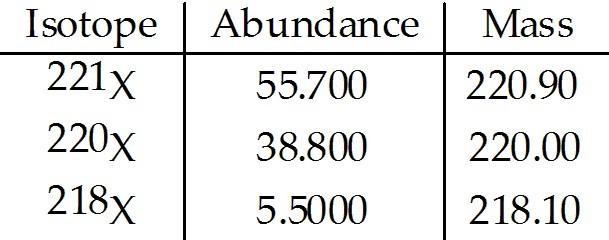 The element X has three naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is ________ amu. A) 33.333 | back 211 B |
front 212 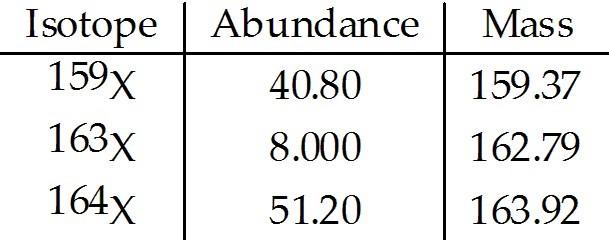 The element X has three naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is ________ amu. A) 159.4 | back 212 B |
front 213 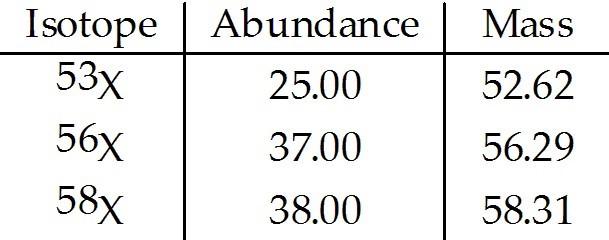 The element X has three naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is ________ amu. A) 52.62 | back 213 B |
front 214 The element ________ is the most similar to helium in chemical and
physical properties. | back 214 D |
front 215 Which pair of elements would you expect to exhibit the greatest
similarity in their physical and chemical properties? | back 215 C |
front 216 Which one of the following is a metalloid? | back 216 E |
front 217 The element lithium is in a group known as the ________. | back 217 E |
front 218 The element chlorine is in a group known as the ________. | back 218 E |
front 219 The element calcium is in a group known as the ________. | back 219 E |
front 220 Of the following, only ________ is not a metalloid. | back 220 B |
front 221 Which of the following elements is a nonmetal? | back 221 A |
front 222 Which one of the following will occur as diatomic molecules in
elemental form? | back 222 C |
front 223 How many electrons does the Al3+ ion possess? | back 223 B |
front 224 How many protons does the Br- ion possess? | back 224 E |
front 225 Which one of the following is most likely to gain electrons when
forming an ion? | back 225 C |
front 226 The formula of a salt is XCl2 . The X-ion in this salt has 24
electrons. The metal X is ________. | back 226 B |
front 227 Predict the charge of the most stable ion of bromine. | back 227 D |
front 228 Predict the charge of the most stable ion of aluminum. | back 228 E |
front 229 Which of the following compounds would you expect to be
ionic? | back 229 D |
front 230 Which species below is the sulfate ion? | back 230 B |
front 231 Which species below is the nitrate ion? | back 231 B |
front 232 Which species below is the chromate ion? | back 232 B |
front 233 The correct name for CaO is ________. | back 233 A |
front 234 Element M reacts with fluorine to form an ionic compound with the
formula M . The M-ion has 21 electrons. Element M is ________. | back 234 B |
front 235 The charge on the copper ion in the salt CuO is ________. | back 235 B |
front 236 The charge on the silver ion in the salt AgCl is ________. | back 236 B |
front 237 The name of the ionic compound NaBrO4 is ________. | back 237 A |
front 238 When a bromine atom forms the bromide ion, it has the same charge as
the ________ ion. | back 238 C |
front 239 Which element forms an ion with the same charge as the sulfate
ion? | back 239 E |
front 240 The correct name for Na2O2 is ________. | back 240 D |
front 241 Which metal is not required to have its charge specified in the names
of ionic compounds it forms? | back 241 D |
front 242 The following hypothetical element : x : can be found in which group on the periodic table? | back 242 VIA |
front 243 Which element is found in Period 2 and Group VIIA? | back 243 fluorine |
front 244 The formula for potassium sulfide is ________. | back 244 K2S |
front 245 What is the name of an alcohol derived from hexane? | back 245 hexanol |
front 246 The possible oxidation numbers for iron are +1 and +2. | back 246 false |
front 247 The formula for chromium (II) iodide is CrI2. | back 247 true |
front 248 H2SeO4 is called selenic acid. | back 248 true |
front 249 The correct name for Na3N is sodium azide. | back 249 false |