Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Central Science: Chapter 19
front 1 The first law of thermodynamics can be given as ________. | back 1 A |
front 2 A reaction that is spontaneous as written ________. | back 2 B |
front 3 Of the following, only ________ is not a state
function. | back 3 C |
front 4 When a system is at equilibrium, ________. | back 4 D |
front 5 A reversible process is one that ________. | back 5 A |
front 6 Which of the following statements is true? | back 6 C |
front 7 The thermodynamic quantity that expresses the extent of randomness in
a system is ________. | back 7 D |
front 8 For an isothermal process, ΔS = ________. | back 8 B |
front 9 Which one of the following is always positive when a spontaneous
process occurs? | back 9 C |
front 10 The entropy of the universe is ________. | back 10 C |
front 11 The second law of thermodynamics states that ________. | back 11 C |
front 12 Which of the following statements is false? | back 12 A |
front 13 Which one of the following processes produces a decrease of the
entropy of the system? | back 13 C |
front 14 Consider a pure crystalline solid that is heated from absolute zero
to a temperature above the boiling point of the liquid. Which of the
following processes produces the greatest increase in the entropy of
the substance? | back 14 E |
front 15 Which one of the following correctly indicates the relationship
between the entropy of a system and the number of different
arrangements, W, in the system? | back 15 D |
front 16 The entropy change accompanying any process is given by the
equation: | back 16 C |
front 17 ΔS is positive for the reaction ________. | back 17 D |
front 18 ΔS is positive for the reaction ________. | back 18 C |
front 19 ΔS is positive for the reaction ________. | back 19 C |
front 20 ΔS is positive for the reaction ________. | back 20 B |
front 21 Which reaction produces a decrease in the entropy of the
system? | back 21 D |
front 22 A decrease in the entropy of the system is observed for the reaction
________. | back 22 D |
front 23 Which reaction produces an increase in the entropy of the
system? | back 23 B |
front 24 Which of the following reactions would have a negative ΔS? | back 24 D |
front 25 ΔS is positive for the reaction ________. | back 25 B |
front 26 For an isothermal process, the entropy change of the surroundings is
given by the equation: | back 26 E |
front 27 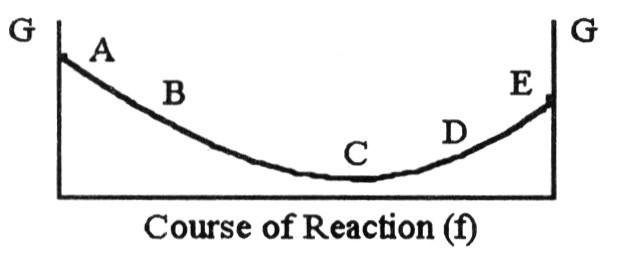 The equilibrium position corresponds to which letter on the graph of
G vs. f (course of reaction) below? | back 27 C |
front 28 For the reaction | back 28 B |
front 29 For the reaction | back 29 A |
front 30 A reaction that is not spontaneous at low temperature can become
spontaneous at high temperature if ΔH is ________ and ΔS is
________. | back 30 A |
front 31 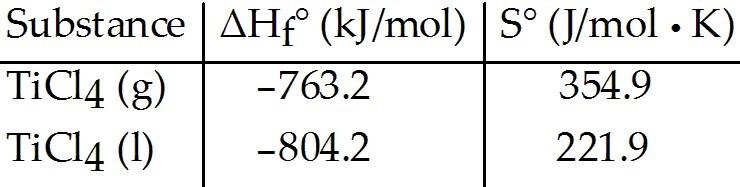 Given the following table of thermodynamic data, complete the
following sentence. The vaporization of TiCl4 is ________. | back 31 C |
front 32 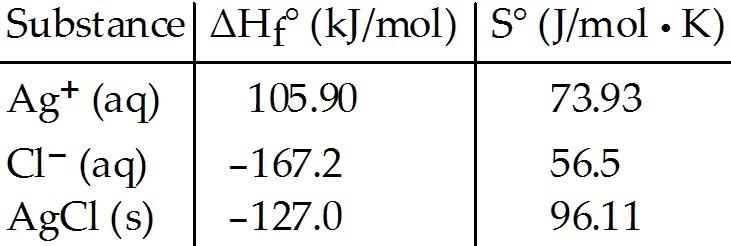 Consider the reaction: Ag+ (aq) + Cl- (aq) → AgCl (s) Given the following table of thermodynamic data, determine the
temperature (in °C) above which the reaction is nonspontaneous under
standard conditions. | back 32 E |
front 33 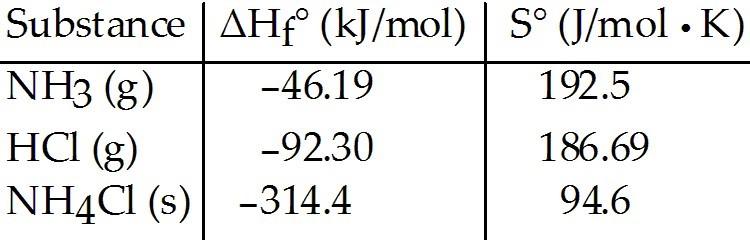 Consider the reaction: NH3 (g) + HCl (g) → NH4Cl (s) | back 33 D |
front 34 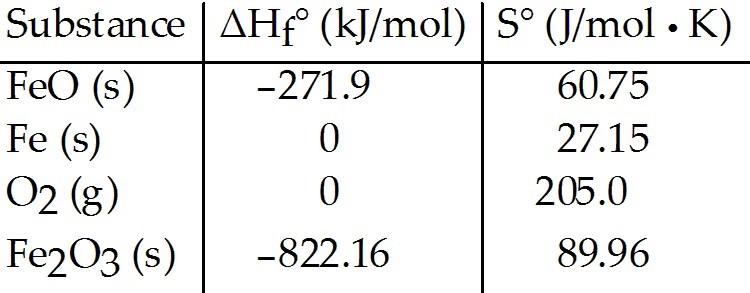 Consider the reaction: FeO (s) + Fe (s) + O2 (g) → Fe2O3 (s) | back 34 B |
front 35 With thermodynamics, one cannot determine ________. | back 35 A |
front 36 Which one of the following statements is true about the equilibrium
constant for a reaction if ΔG° for the reaction is negative? | back 36 C |
front 37 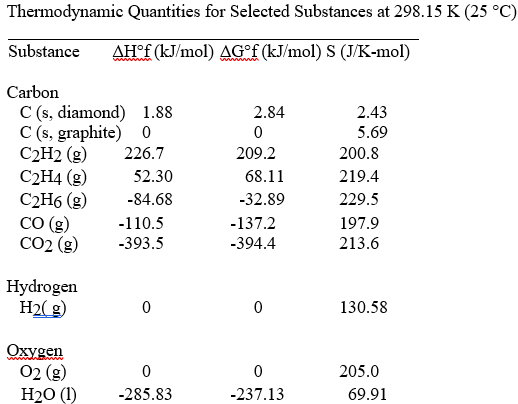 The value of ΔS° for the catalytic hydrogenation of acetylene to ethene, C2H2 (g) + H2 (g) → C2H4 (g) is ________ J/K∙ mol. | back 37 D |
front 38  The combustion of acetylene in the presence of excess oxygen yields carbon dioxide and water: 2C2H2 (g) + 5O2 (g) → 4CO2 (g) + 2H2O (l) The value of ΔS° for this reaction is ________ J/K ∙ mol. | back 38 E |
front 39  The value of ΔS° for the reaction 2C (s, diamond) + O2 (g) → 2CO (g) is ________ J/K ∙ mol. | back 39 B |
front 40  The value of ΔS° for the catalytic hydrogenation of ethene to ethane, C2H4 (g) + H2(g) → C2H6 (g) is ________ J/K ∙ mol. | back 40 B |
front 41  The value of ΔS° for the catalytic hydrogenation of acetylene to ethane, C2H2 (g) + 2H2 (g) → C2H6 (g) is ________ J/K ∙ mol. | back 41 C |
front 42  The value of ΔS° for the oxidation of carbon to carbon monoxide, 2C (s, graphite) + O2 (g) → 2CO (g) is ________ J/K ∙ mol. Carbon monoxide is produced in the
combustion of carbon with limited oxygen. | back 42 D |
front 43  The value of ΔS° for the oxidation of carbon to carbon dioxide, C (s, graphite) + O2 (g) → CO2(g) is ________ J/K ∙ mol. The combustion of carbon, as in charcoal
briquettes, in the presence of abundant oxygen produces carbon
dioxide. | back 43 E |
front 44  The combustion of ethene in the presence of excess oxygen yields carbon dioxide and water: C2H4 (g) + 3O2 (g) → 2CO2 (g) + 2H2O (l) The value of ΔS° for this reaction is ________ J/K ∙ mol. | back 44 A |
front 45  The combustion of ethane in the presence of excess oxygen yields carbon dioxide and water: 2C2H6 (g) + 7O2 (g) → 4CO2 (g) + 6H2O (l) The value of ΔS° for this reaction is ________ J/K ∙ mol. | back 45 B |
front 46  The combustion of hydrogen in the presence of excess oxygen yields
liquid water: What is the value of ΔS° in J/K ∙ mol. for this
reaction? | back 46 D |
front 47  The value of ΔS° for the oxidation of solid elemental sulfur to gaseous sulfur trioxide, 2S (s, rhombic) + 3O2(g) → 2SO3 (g) is ________ J/K ∙ mol. | back 47 D |
front 48  The value of ΔS° for the oxidation of solid elemental sulfur to gaseous sulfur dioxide, S (s, rhombic) + O2(g) → SO2(g) is ________ J/K ∙ mol. | back 48 E |
front 49  The value of ΔS° for the decomposition of gaseous sulfur trioxide to solid elemental sulfur and gaseous oxygen, 2SO3 (g) → 2S (s, rhombic) + 3O2 (g) is ________ J/K ∙ mol. | back 49 D |
front 50  The value of ΔS° for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen, SO2 (g) → S (s, rhombic) + O2 (g) is ________ J/K ∙ mol. | back 50 C |
front 51  The value of ΔS° for the formation of POCl3 from its constituent elements, P2 (g) + O2 (g) + 3Cl2 (g) → 2POCl3 (g) is ________ J/K ∙ mol. | back 51 A |
front 52  The value of ΔS° for the decomposition of POCl3 into its constituent elements, 2POCl3 (g) → P2 (g) + O2 (g) + 3Cl2 (g) is ________ J/K ∙ mol. | back 52 B |
front 53  The value of ΔS° for the formation of phosphorous trichloride from its constituent elements, P2 (g) + 3Cl2 (g) → 2PCl3 (g) is ________ J/K ∙ mol. | back 53 C |
front 54  The value of ΔS° for the decomposition of phosphorous trichloride into its constituent elements, 2PCl3 (g) → P2 (g) + 3Cl2( g) is ________ J/K ∙ mol. | back 54 C |
front 55  The value of ΔS° for the formation of calcium chloride from its constituent elements, Ca (s) + Cl2 (g) → CaCl2 (s) is ________ J/K ∙ mol. | back 55 D |
front 56  The value of ΔS° for the decomposition of calcium chloride into its constituent elements, CaCl2 (s) → Ca (s) + Cl2 (g) is ________ J/K ∙ mol. | back 56 E |
front 57  The value of ΔH° for the oxidation of solid elemental sulfur to gaseous sulfur trioxide, 2S (s, rhombic) + 3O2( g) → 2SO3 (g) is ________ kJ/mol. | back 57 B |
front 58  The value of ΔH° for the decomposition of gaseous sulfur trioxide to its component elements, 2SO3 (g) → 2S (s, rhombic) + 3O2 (g) is ________ kJ/mol. | back 58 A |
front 59  The value of ΔH° for the oxidation of solid elemental sulfur to
gaseous sulfur dioxide, is ________ kJ/mol. | back 59 B |
front 60  The value of ΔH° for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen, SO2 (g) → S (s,rhombic) + O2 (g) is ________ kJ/mol. | back 60 E |
front 61  The value of ΔH° for the formation of POCl3 from its constituent elements, P2 (g) + O2 (g) + 3Cl2 (g) → 2POCl3 (g) is ________ kJ/mol. | back 61 A |
front 62  The value of ΔH° for the decomposition of POCl3 into its constituent elements, 2POCl3 (g) → P2 (g) + O2 (g) + 3Cl2 (g) is ________ kJ/mol. | back 62 B |
front 63  The value of ΔH° for the formation of phosphorous trichloride from its constituent elements, P2 (g) + 3Cl2 (g) → 2PCl3 (g) is ________ kJ/mol | back 63 C |
front 64  The value of ΔH° for the decomposition of phosphorous trichloride into its constituent elements, 2PCl3 (g) → P2 (g) + 3Cl2 (g) is ________ kJ/mol. | back 64 C |
front 65  The value of ΔH° for the formation of calcium chloride from its constituent elements, Ca (s) + Cl2 (g) → CaCl2 (s) is ________ kJ/mol. | back 65 D |
front 66  The value of ΔH° for the decomposition of calcium chloride into its constituent elements, CaCl2 (s) → Ca (s) + Cl2 (g) is ________ kJ/mol. | back 66 E |
front 67  The value of ΔG° at 25 °C for the oxidation of solid elemental sulfur to gaseous sulfur trioxide, 2S (s, rhombic) + 3O2 (g) → 2SO3 (g) is ________ kJ/mol. | back 67 D |
front 68  The value of ΔG° at 25 °C for the oxidation of solid elemental sulfur to gaseous sulfur dioxide, S (s, rhombic) + O2(g) → SO2 (g) is ________ kJ/mol. | back 68 E |
front 69  The value of ΔG° at 25 °C for the decomposition of gaseous sulfur trioxide to solid elemental sulfur and gaseous oxygen, 2SO3 (g) → 2S (s, rhombic) + 3O2 (g) is ________ kJ/mol. | back 69 A |
front 70  The value of ΔG° at 25 °C for the decomposition of gaseous sulfur
dioxide to solid elemental sulfur and gaseous oxygen, is ________ kJ/mol. A) +395.2 | back 70 D |
front 71  The value of ΔG° at 25 °C for the formation of POCl3 from its constituent elements, P2 (g) + O2 (g) + 3Cl2 (g) → 2POCl3 (g) is ________ kJ/mol. | back 71 A |
front 72  The value of ΔG° at 25 °C for the decomposition of POCl3 into its constituent elements, 2POCl3 (g) → P2 (g) + O2 (g) + 3Cl2 (g) is ________ kJ/mol. | back 72 B |
front 73  The value of ΔG° at 25 °C for the formation of phosphorous trichloride from its constituent elements, P2 (g) + 3Cl2 (g) → 2PCl3 (g) is ________ kJ/mol. | back 73 C |
front 74  The value of ΔG° at 25 °C for the decomposition of phosphorous trichloride into its constituent elements, 2PCl3 (g) → P2 (g) + 3Cl2 (g) is ________ kJ/mol. | back 74 D |
front 75  The value of ΔG° at 25 °C for the formation of calcium chloride from its constituent elements, Ca (s) + Cl2 (g) → CaCl2 (s) is ________ kJ/mol. | back 75 E |
front 76  The value of ΔG° at 25 °C for the decomposition of calcium chloride into its constituent elements, CaCl2 (s) → Ca (s) + Cl2 (g) is ________ kJ/mol. | back 76 D |
front 77  The value of ΔG° at 373 K for the oxidation of solid elemental sulfur
to gaseous sulfur dioxide is ________ kJ/mol. At 298 K, ΔH° for this
reaction is -269.9 kJ/mol, and ΔS° is +11.6 J/K. | back 77 B |
front 78  The value of ΔG° at 25 °C for the following reaction: C2H4 (g) + H2 (g) → C2H6 (g) is ________ kJ/mol. At 298 K, ΔH° for this reaction is -137.5
kJ/mol, and ΔS° is +120.5 J/K. | back 78 B |
front 79 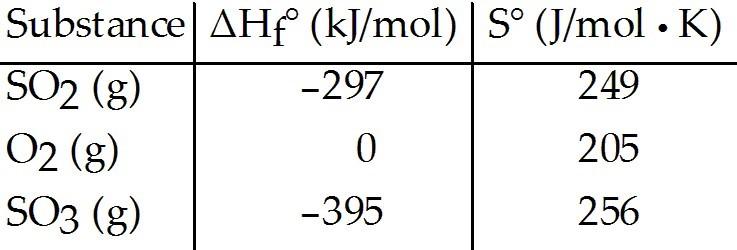 Given the thermodynamic data in the table below, calculate the
equilibrium constant (at 298 K) for the reaction: A) 2.40 × 1024 | back 79 A |
front 80 The value of ΔG° for a reaction conducted at 25 °C is 3.05 kJ/mol.
The equilibrium constant for a reaction is ________ at this
temperature. | back 80 A |
front 81 What is the equilibrium constant for a reaction at 25 °C? ΔG° for the
reaction is 2.8 kJ/mol. | back 81 B |
front 82 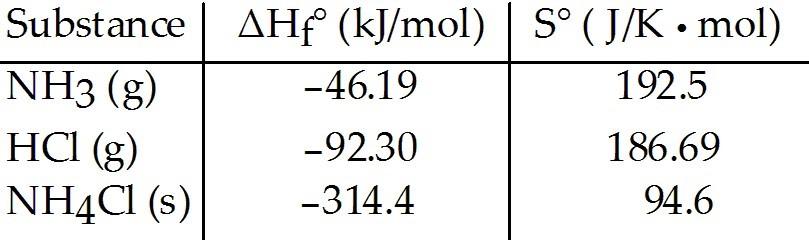 Consider the reaction between ammonia and hydrochloric acid to
produce ammonium chloride. The value of K for the reaction at 25 °C is ________. | back 82 D |
front 83 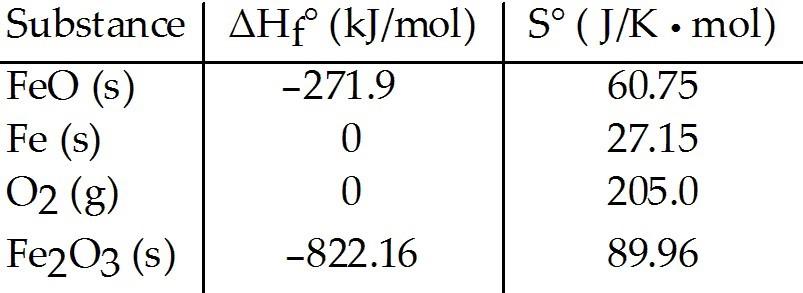 Consider the reaction: FeO (s) + Fe (s) + O2(g) → Fe2O3 (s) The value K for the reaction at 25 °C is ________. | back 83 D |
front 84 Consider the formation of solid silver chloride from aqueous silver and chloride ions. Given the following table of thermodynamic data at 298 K: | back 84 E |
front 85 The normal boiling point of methanol is 64.7°C and the molar enthalpy
of vaporization if 71.8 kJ/mol. The value of ΔS when 1.75 mol of
CH3OH(I) vaporizes at 64.7 °C is ________ J/K | back 85 B |
front 86 The normal boiling point of water is 100.0 °C and its molar enthalpy
of vaporization is 40.67 kJ/mol. What is the change in entropy in the
system in J/K when 24.7 grams of steam at 1 atm condenses to a liquid
at the normal boiling point? | back 86 C |
front 87 The normal boiling point of C2Cl3F3 is 47.6 °C and its molar enthalpy
of vaporization is 27.49 kJ/mol. What is the change in entropy in the
system in J/K when 28.6 grams of C2Cl3F3 vaporizes to a gas at the
normal boiling point? | back 87 D |
front 88 What is the change in entropy in the system in J/K when 112.2 grams
of ethanol at 1 atm condenses to a liquid at the normal boiling point?
The normal boiling point of ethanol (C2H5OH) is 78.3 °C and its molar
enthalpy of vaporization is 38.56 kJ/mol. | back 88 C |
front 89 Which one of the following processes produces a decrease in the
entropy of the system? | back 89 A |
front 90 ΔS is negative for the reaction ________. | back 90 A |
front 91 Which of the following has the largest entropy? | back 91 A |
front 92 Which of the following has the largest entropy? | back 92 A |
front 93 Which of the following has the largest entropy at 25 °C and
atm? | back 93 C |
front 94 Which of the following has the largest entropy at 25 °C and
atm? | back 94 C |
front 95 The standard Gibbs free energy of formation of ________ is zero. (a) H2O (l) A) (a) only | back 95 C |
front 96 The standard Gibbs free energy of formation of ________ is zero. (a)H2 O (l) A) (a) only | back 96 D |
front 97 The standard Gibbs free energy of formation of ________ is zero. (a) Mn (s) A) (a) only | back 97 E |
front 98 The value of ΔG° at 261.0 °C for the formation of phosphorous trichloride from its constituent elements, P2(g) +3CL2 (g) → 2PCL3(g) is ________ kJ/mol. At 25.0 °C for this reaction, ΔH° is -720.5
kJ/mol, ΔG° is -643.9 kJ/mol, and ΔS° is -263.7 J/K | back 98 A |
front 99 The value of ΔG° at 181.0 °C for the formation of calcium chloride
from calcium metal and chlorine gas is ________ kJ/mol. At 25.0 °C for
this reaction, ΔH° is -795.8 kJ/mol, ΔG° is -748.1 kJ/mol, and ΔS° is
-159.8 J/K | back 99 D |
front 100 The signs of ΔH° and ΔS° must be ________ and ________, respectively,
for a reaction to be spontaneous at high temperatures but
nonspontaneous at low temperatures. | back 100 D |
front 101 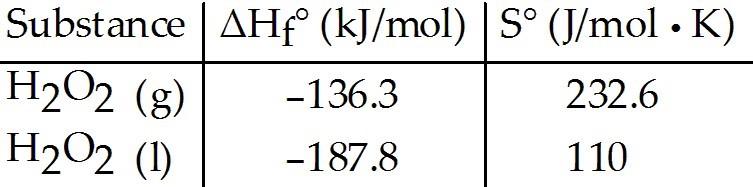 Given the following table of thermodynamic data, complete the
following sentence. The vaporization of H2O2 (l) is ________. | back 101 A |
front 102 For the reaction ΔH° = 133.3 kJ/mol and ΔS° = 121.6 J/K ∙ mol at 298 K. At
temperatures greater than ________ °C this reaction is spontaneous
under standard conditions. | back 102 D |
front 103 At what temperature in Kelvin will a reaction have ΔG = 0? ΔH = -24.2
kJ/mol and ΔS = -55.5 J/K-mol and assume both do not vary with
temperature. | back 103 D |
front 104 At what temperature will a reaction be spontaneous? ΔH = +22.2 kJ/mol
and ΔS = +81.1 J/K-mol and assume both do not vary with
temperature. | back 104 D |
front 105 For a given reaction, ΔH = +74.6 kJ/mol, and the reaction is
spontaneous at temperatures above the crossover temperature, 449 K.
The value of ΔS = __________ J/mol ∙ K, assuming that ΔH and ΔS do not
vary with temperature. | back 105 A |
front 106 For a given reaction, ΔS = +69.0 J/mol∙K, and the reaction is
spontaneous at temperatures above the crossover temperature, 439 K.
The value of ΔH = __________ kJ/mol, assuming that ΔH and ΔS do not
vary with temperature. | back 106 A |
front 107 For a given reaction with ΔH = -28.1 kJ/mol, the ΔG = 0 at 372 K. The
value of ΔS must be __________ J/K-mol, assuming that ΔH and ΔS do not
vary with temperature. | back 107 A |
front 108 For a given reaction with ΔS = -50.8 J/K-mol, the ΔG = 0 at 395 K.
The value of ΔH must be __________ kJ/mol, assuming that ΔH and ΔS do
not vary with temperature. | back 108 A |
front 109 What is the equilibrium constant for a reaction at 25 °C. The value
of ΔG° is -57.5 kJ/mol. | back 109 D |
front 110 If ΔG° for a reaction is less than zero, then ________. | back 110 A |
front 111 In the Haber process, ammonia gas is synthesized from nitrogen gas
and hydrogen gas. ΔG° at 298 K for this reaction is -33.3 kJ/mol. The
value of ΔG at 298 K for a reaction mixture that consists of 1.9 atm
nitrogen gas, 2.3 atm hydrogen gas, and 0.85 atm ammonia gas is
________. | back 111 E |
front 112 Phosphorous and chlorine gases combine to produce phosphorous
trichloride. ΔG° at 298 K for this reaction is -642.9 kJ/mol. The
value of ΔG at 298 K for a reaction mixture that consists of 1.9 atm
P2, 1.6 atm CL2, and PCL3 is ________. | back 112 A |
front 113 The equilibrium constant for a reaction is 0.38 at 25 °C. What is the
value of ΔG° (kJ/mol) at this temperature? | back 113 A |
front 114 The equilibrium constant for the following reaction is 3.0 ×
108 at 25 °C. The value of ΔG° for this reaction is ________ kJ/mol. | back 114 D |
front 115 A reversible change produces the maximum amount of ________ that can be achieved by the system on the surroundings. | back 115 work |
front 116 Calculate ΔG° (in kJ/mol) for the following reaction at 1 atm and 25 °C: C2H6 (g) + O2 (g) → CO2 (g) + H2O (l) (unbalanced) ΔGf° C2H6 (g) = -32.89 kJ/mol; ΔGf° CO2 (g) = -394.4 kJ/mol; ΔGf° H2O (l) = -237.13 kJ/mol | back 116 -2935.0 |
front 117 Calculate ΔG∘ (in kJ/mol) for the following reaction at 1 atm and 25 °C: C2H6 (g) + O2 (g) → CO2 (g) + H2O (l) (unbalanced) ΔHf∘ C2H6 (g) = -84.7 kJ/mol; S∘ C2H6 (g) = 229.5 J/K ∙ mol;
| back 117 -2934.0 |
front 118 At what temperature (in K) will a reaction become spontaneous? ΔH is 115.0 kJ/mol and ΔS is 75.00 J/K ∙ mol. | back 118 1533 |
front 119 At what temperature (in K) will a reaction become spontaneous? ΔH is 65.0 kJ/mol and ΔS is 149.00 J/K ∙ mol. | back 119 436 |
front 120 What is the ΔG° (kJ/mol) for the formation of silver chloride at 25 °C? Ksp = 1.8 × 10-10 | back 120 56 |
front 121 The melting of a substance at its melting point is an isothermal process. | back 121 true |
front 122 The vaporization of a substance at its boiling point is an isothermal process. | back 122 true |
front 123 The quantity of energy gained by a system equals the quantity of energy gained by its surroundings. | back 123 false |
front 124 The entropy of a pure crystalline substance at 0 K is zero. | back 124 true |
front 125 The more negative ΔG° is for a given reaction, the larger the value of the corresponding equilibrium constant, K. | back 125 true |