Biology 1406 Final Review
What is the term for metabolic pathways that release stored energy by breaking down complex molecules?
Catabolic pathways
The molecule that functions as a reducing agent (electron donor) in a redox or oxidation-reduction reaction
Loses electrons and loses potential energy
Which of the following statements describes the result of this reaction. C6H12O6 + 6O2 --> 6 CO2 + 6 H2O
C6H12O6 is oxidized and O2 is reduced
Which term describes the degree to which an element attracts electrons?
Electronegativity
The oxygen consumed during cellular respiration is involved directly in which process or event?
Accepting electron at the end of the electron transport chain.
In glycolysis ATP molecules are produced by
Substrate level phosphorylation
During glycolysis, when each molecule of glucose is catabolized to two molecules of pyruvate, most of the potential energy contained in glucose is
Pretained in pyruvate
Which statement about the citric acid cycle is correct?
The last reaction in the citric acid cycle produces a product that is a substrate for the first reaction of the citric acid cycle
Which of the following statements about the chemiosmotic synthesis of ATP Is correct?
The chemiosmotic synthesis of ATP requires that the electron transport be coupled to proton transport.
In alcohol fermentation NAD+ is regenerated from NADH by
Reduction of acetaklehyde to ethanol
Cytosine makes up 42% of nucleotides, how much does thymine make up?
8% thymine
Because most receptors are membrane proteins, which is true about how they act?
They change their conformation after binding w/ signal polypeptides
Which of the following provides molecular evidence that signal transduction evolved?
The molecular of cell signaling are quite common in ancestors was a billion years ago
Histamine is a chemical substance released in inflammatory and allergenic responses. Histamine diffuses across the plasma membrane to bind to the H1 receptor inside the cell. When a neuron responds to a particular neurotransmitter by opening gated ion, neurotransmitter is
Signal molecule
One of the major categories of receptors in plasma membranes react by forming dimers.
Receptor tyrosine kinase
Correct association:
GTPase activity and hydrolysis of GTP to GDP
Most common second messenger:
Calcium & Camp
Best explanation for most transduction pathways have multiple steps:
Multiple steps provide greater possible amplification of a signal.
Function of phosphatases in signal transduction:
Inactivate protein kinase and turn off signal transduction
Centromere is a region:
Chromatids remain attached to 1 another
Starting w/ a fertilized egg (zygote) 5 cell division
32
Apoptosis is potentially threatening to neighboring cells because:
Lysosomal enzymes exiting dying cell would damage surrounding cells
Lipid - soluble signaling molecules,cross the membrane but affect only target cells because.
Intracellular receptors are present only in target cells
MOVEMENT of chromosomes during anaphase would be affected by a drug that.
Prevents shortening of microtubules.
Why do chromosomes coil during mitosis ?
To allow the chromosome to move without becoming entangled and breaking
Effects on a nonsense mutation gene
It introduces a premature stop codon into the MRNA
Proteins involved in regulation of cell cycle and shows fluctuations in concentration is referred to as what?
Cyclins
Besides the ability of some cancer cells to over proliferate..
Lack of appropriate cell deaths
Which of the following does not occur in prokaryotic gene expression, but does in eukaryotic gene expression.
A poly tails is added to the 3 end of an MRNA and a cap is added to the 5 end.
What is a ribozyme?
An enzyme that catalyzes the association between the large and small ribosomal subunits
AAA anticodon on TRNA
AAA
When the function of the newly made polypeptide is to be secreted from the cell where is has been made, what must occur?
It's signal sequence must target it to the ER, from which it goes to the Golgi.
High levels of citric acid inhibit the enzyme phosphofructokinase, a key enzyme in glycolysis. Citric acid binds to the enzyme at a different location from the active site. This is an example of
Allosteric regulation
What is the importance of the light-independent reactions of photosynthesis in terms of carbon flow in the biosphere?
The light-independent reactions turn CO2 Into usable carbon in the form of sugars.
Which of the following statements best describes the relationship Btwn the light-dependent and light-independent reactions of photosynthesis?
The light-dependent reactions produce ATP AND NADPH, which are then used by light-independent reactions.
Which of the following reactions ensures that the Calvin cycle can make a continuous supply of glucose
Regeneration of RuBP
C4 plants occur more commonly in desert conditions because
The stomata open at night and close in the day
Select the correct statement about the Calvin cycle.
The Calvin cycle has 3 phases: Carbon fixation, reduction,and regeneration of RuBP
Which of the following best describes the structure of a biological membrane?
Two layers of phospholipids with protein either crossing the layers or on the surface of the layers.
Celery stalks that are immersed in fresh water for several hours become stiff and hard. Similar stalks in 0.15 M salt solution become limp and soft. From this we can deduce that the cells of the celery stalk..
Hypertonic to fresh water but hypotonic to the salt solution.
Which line in the graph represents the bag that contained a solution isotonic to the 0.6 M solution at the beginning of the experiment?
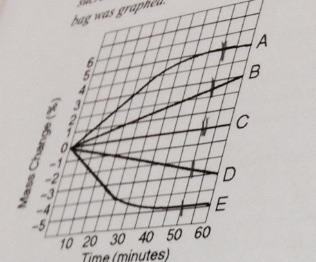
C
Which line in the graph represents the bag with the highest initial concentration of sucrose?
A
Which line or lines in the graph represents bags that contain a solution that is still hypertonic at 50 minutes?
B
Which of the following would likely move through a lipid bilayer of a plasma membrane most rapidly?
CO2
The primary function of polysaccharides attached to glycoproteins and glycolipids of animal cell membranes is....
To mediate cell to cell recognition
In facilitated diffusion, what is the role of the transport protein?
Transport proteins provide a hydrophilic route for the solute to cross the membrane
What distinguishes facilitated diffusion from simple diffusion?
Membrane proteins help move molecules across the membrane.
Lactose transport but membrane proteins occurs under conditions in which the concentration of lactose inside the cell is higher than the concentration outside the cell. What type of transport is used to move lactose into the cell?
Active transport
Endocytosis moves materials ___ a cell via __
Into......membranous vesicles
Which of the following factors would tend to increase membrane fluidity?
A greater proportion of unsaturated phospholipids
Which of the following statements about equilibrium of chemical reactions is correct?
A reaction that is at equilibrium is not capable of doing any work
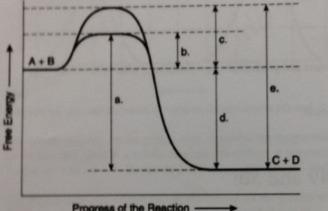
Which of the following terms best describes the forward reaction in figure 8.1?
Exergonic, G< 0
Which of the following will be the same either an enzyme catalyzed or noncatalyze reaction
D
Which of the following represents the difference between the free energy content of the reactants and energy content of the products in the figure?
D
Which of the following represents the activation energy required for the enzyme catalyzed reaction in the figure?
B
In cells, what is usually the immediate source of energy for it in or going to reaction?
ATP
All of the following are part of a prokaryotic cell wall except
Mitochondria
Synthesis of oils, phospholipids,steroids?
Smooth ER
Why are human sex hormones categorized as lipids?
Not soluble in water
Light reactions of photosynthesis use __and produce ___
Water & NADPH
Control for an experiment
control group is matched with experiment group except for the one experimental variable
correctly describes equilibrium?
forward and reverse reaction continues with no effect on the concentrations of the reactants and products
what is the atomic number of cation formed in the illustration above?
11
if the ph of a solution is increased from 5 to 7 it means that the concentration of H+ is?
one-hundredth 1/100 what it was at ph 5
polysaccaharides, triglycerides, and proteins are similar in that they-
are synthesized from monomers by the process of hydrolysis
if DNA has the nitrogen base sequence 5' ATTTGC 3'
3' GCAAAT 5'
all of the following are part of a prokaryotic cell except?
Cell wall
hydrolytic enzymes must be segregated and packaged to prevent general destruction of cellular components. Which of the following organelles contain these hydrolytic enzymes in animal cells?
lysosome
the volume enclosed by the plasma membrane of plant cells is often much larger than the corresponding volume in animal cells. The most reasonable explanation:
plant cells contain a large vacuole that reduces the volume of the cytoplasm
the evolution of eukaryotic cells most likely involved:
endosymbiosis of an aerobic bacterium in a larger host cell , the endosym. evolved into mitochondria
A cell has the following molecules and structures: enzymes, DNA,ribosomes, plasma membrane, and mitochondria. It could be a cell from
nearly any eukaryotic organism
All proteins are synthesized by ribosomes in the cell. Some ribosomes
float freely in the cytosol, while others are bound to the surface of
the endoplasmic reticulum. Most proteins made by free ribosomes
function in the cytosol. Proteins made by bound ribosomes either
function within the endomembrane system or pass through it and are
secreted from the cell.
Which of the following proteins are
synthesized by bound ribosomes?
insulin
if a solution has a ph of 7, this means?
the concentration of H+ ions in the water equals the concentration of OH- ions in the water
how many electron pairs does carbon share in order to complete its valence shell?
4
A carbon atom is most likely to form what kind of bond with other atoms?
covalent
the figure above shows glucose and fructose, how do they differ?
arrangement of carbon, hydrogen, and oxygen atoms.
Fructose and Glucose
structural isomers
what is the chemical reaction mechanism by which cells make polymers from monomers?
dehydration reactions
characteristics of alcohols?
-OH
which functional group shown are present in all amino acids?
-NH2
which group is carboxyl functional group?
O
=
-C-O-H
___ structure is the sequence of amino acids in a protein
primary
__structure is achieved when a protein folds into a compact, three-dimensional shape stabilized by interactions between side-chain "R-groups" of amino acids.
Tertiary
__structure is the result of 2 or more protein subunits assembling to form a larger, biologically active protein complex.
quartermary
__ structure describes the alpha-helices and beta-sheets that are formed by hydrogen bonding between backbone atoms located near each other in the polypeptide chain.
secondary
Identify a possible set of components of DNA nucleotide:
deoxyribose, phosphate group, thymine
what # and types of chromosomes are found in a human somatic cell?
44 autosomes and 2 sex chromosomes
Eukaryotic sexual life cycles show tremendous variation. Which do all sexual life cycles have in common?
II, III, IV Meiosis, Fertilization, and Gametes
If snap dragons are heterozygous for flower color, mating btwn them will result in what ratio?
1:2:1
Height in humans generally shows a normal (bell-shaped) distribution. What type of inheritance most likely determines height?
combination of polygenic inheritance and environmental factors
An obstetrician knows
that one of her patients is a pregnant woman whose fetus is at risk
for a serious disorder
that is detectable
biochemically in fetal cells. The obstetrician would most reasonably
offer which of the
following procedures to
her patient
Aminocentesis
What is the genotype of the deceased individual in generation 2?
heterozygous for a gene for colon cancer
how does this trait seem to be inherited?
an autosomal dominant
Protein produced by a regulatory gene?
repressor
Transcription of structural genes in an audible operon--
starts when pathway substrate is present
Prokaryotic and Eukaryotic have
Ribosomes in common
Correctly describe chemical equilibrium
Forward and reverse reactions continue with no effect on the concentrations of the reactants and products
if the ph of a solution is increased from ph5 to ph7 it means that
the concentration of the H+ is 1/100 than what it was at ph5
The volume enclosed by the plasma membrane of plant cells is often much larger than the corresponding volume in animals cells the most reasonable explanation for this is that plant cells
contain a large vacuole that reduces the volume of the cytoplasm
The evolution of eukaryotic cells most likely involved endosymbiosis of an aerobic bacterium in a larger host cell
the endosymbio evolved into mitochondria
a cell has the following molecules and structures
enzymes, dna, ribosomes, plasma membrane, and mitochondria it could be a cell from nearly any eukaryotic organism.
All proteins are synthesized by ribosomes in the cell some ribosomes float freely in the cytosol while others are bound to the surface of the ER. Which proteins are synthesized by bound ribsomes ?
Insulin
pathway
ER---> GOLGI-----> VESICLES THAT FUSE WITH THE PLASMA MEMBRANE
Which structure is common to plant and animal cells
Mitochondria
Which structure function pair is mismatched
Microtubule: Muscle contraction
Dna is composed of building blocks called
Nucleotides
order of hierarchy
Molecule, Organelle, Cell, Tissue, Organ, Organ System, Organism, Population, Community, Ecosystem
Organisms produce too many Offspring
and resources are limited
Not True
They must occur under carefully controlled condition found in a laboratory
A controlled experiment is one that tests
experimental and control groups in parallel
All living things share a common genetic language of Dna
because they share a common ancestry
Best Describes Model Organism
it is well studied, easy to grow, and results are widely applicable
Best description of a control for an experiment
Control group is matched with the experimental group except for the one experimental variable
25 of the 92 natural elements are known to be essential to life: which 4 of the 25 make 96% of living matter
Carbon hydrogen nitrogen oxygen
atomic number of neon is 10 which is correct of neon
it has 8 electrons in its outer electron shell and is inert
a covalent chemical bond is one in which
outer shell electrons of 2 atoms are shared so as to fill the outer electron shells of both atoms
what results from unequal sharing of electron between atoms
polar covalent Bond
Most polar Bond
H20
IN A SINGLE MOLECULE OF WATER, 2 HYDROGEN ATOMS ARE BONDED TO A
SINGLE OXYGEN ATOM BY POLAR COVALENT BONDS
water molecules are able to form hydrogen bonds with compounds that have
polar covalent bonds
which type of bond must be broken for water to vaporize
hydrogen bonds
Measurement show that ph of a particular lake is 4.0 what is the hydrogen ion concentration
10-4
if the ph of a solution has increased from 5 to 7 it means that the concentration of H+ is
1/100 what it was at ph5
Carbon shares
4 to complete valence shell
A carbon atom is most likely to form what kind of bond
Covalent
Glucose and fructose are structural isomers meaning they are molecular differ in the
Arrangements of carbon hydrogen and oxygen atoms
True Statement
In sexual reproduction, indivudials transmit half of their nuclear genes to each of their offspring
what number and types are found in human somatic
44 autosomes 2 sex chromosomes
define genome
complete set of organisms gene
x and y chromosomes include genes that determine an individuals sex
meiosis, fertilization, gametes
is what we all do ?
if a cell has completed the 1st meoiotic divisions and is just beginning meosis 2
it has half the amount of DNA as the cell that begun meiosis
human somatic cells are
diploid
which statement best represents the connection between reproduction and revolution
sexual reproduction increases genetic variation because random mutations can be shuffled btwn organism
purple floweres are dom over white
cross the purple flowered plant with white
determined that the original plant was heterozygous
law of segregation