Acids and Bases - Conductivity
HCl

Strong Molecular Acid
Fully Dissociates
Lots of ions
Good electrical Condutivity
HBr
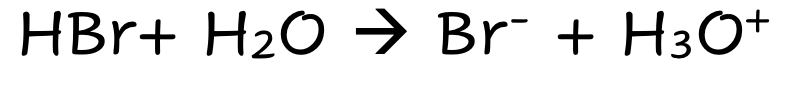
Strong Molecular Acid
Fully Dissociates
Lots of ions
Good electrical Condutivity
HNO3
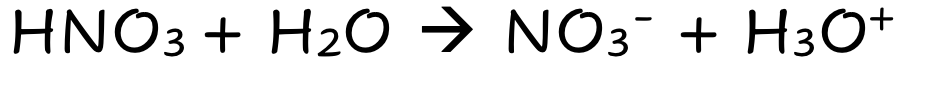
Strong Molecular Acid
Fully Dissociates
Lots of ions
Good electrical Condutivity
CH3COOH
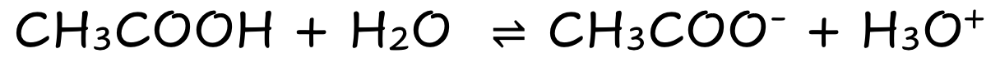
Weak Molecular Acid
Partially Dissociates
Few ions
Poor electrical Condutivity
HF
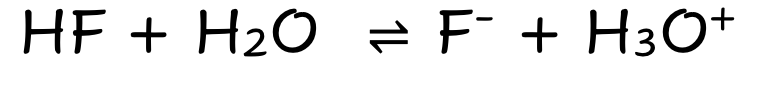
Weak Molecular Acid
Partially Dissociates
Few ions
Poor electrical Condutivity
NH3
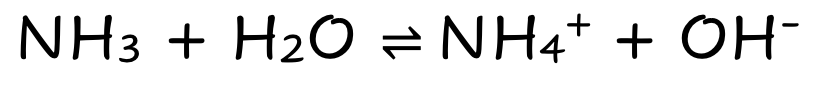
Weak Molecular Base
Partially Dissociates
Few ions
Poor electrical Condutivity
KOH
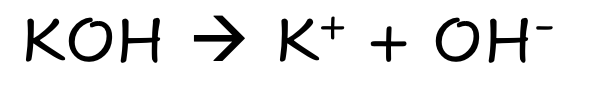
Strong Ionic Base
Fully Dissolves
Lots of ions (ionic species)
Good electrical Condutivity
NaOH

Strong Ionic Base
Fully Dissolves
Lots of ions (ionic species)
Good electrical Condutivity
NaCl

Neutral Ionic Salt
Fully Dissolves
NH4Cl
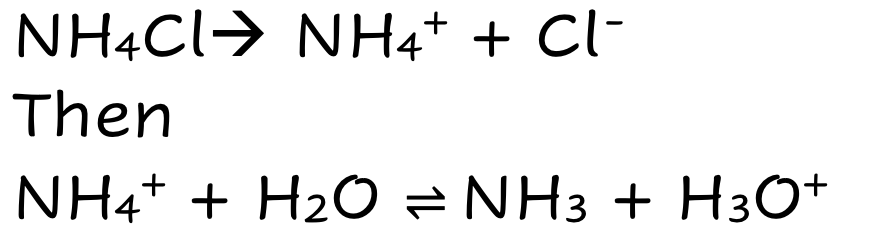
Weak Ionic Acid
Fully Dissolves
THEN
Partially Dissociates
Lots of ions (ionic species)
Good electrical Condutivity
CH3COONa
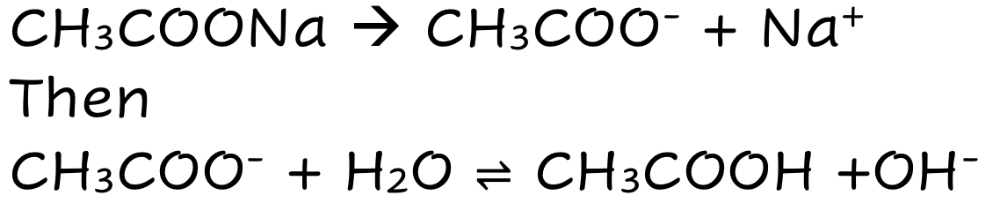
Weak Ionic Base
Fully Dissolves
THEN
Partially Dissociates
Lots of ions (ionic species)
Good electrical Condutivity
CH3CH2OH
Molecular and Neutral
Disolves in water
No Ions in solution
No electrical Condutivity