Prelab for VSEPR SD
Find the number of valence electrons in H Cl. For counting purposes with Lewis structures, the number of valence electrons in an atom of a main group element is equal to the last digit in the group number (or the roman numeral) of that element in the Periodic Table.
In H C l there is a total of ____________ valence electrons.
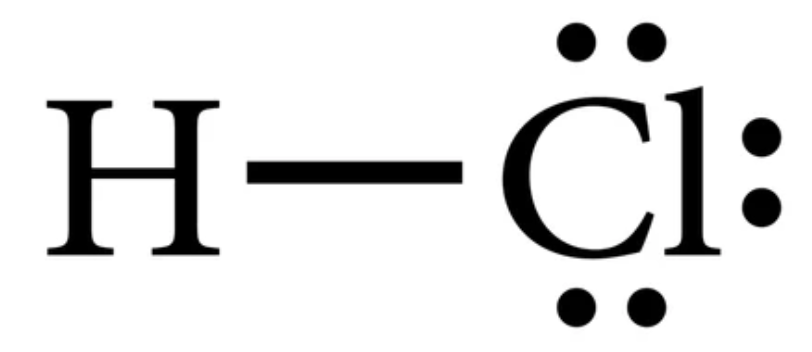
8
The H C l Lewis structure consists of bonds and lone (non-bonding) pairs of electrons. How many bonds (needing 2 electrons) and lone pairs (also needing 2 electrons) are in the Lewis structure for H C l? ____________
4
How many bonds are present in the Lewis structure for the H C l molecule?
1
How many non-bonding electron pairs are in the Lewis structure for the H C l molecule?
3
What is the shape the atoms make (excluding lone pairs) in H C l?
Linear