Postlab for Titration of an Antacid SD
A student performs this experiment but forgets to remove an air bubble from the N a O H buret valve. During the course of the titration, the air bubble disappeared.
Would the measured volume of N a O H added in the back-titration be incorrectly high, incorrectly low, or will the volume remain unchanged?
- remains unchanged
- incorrectly low
- incorrectly high
incorrectly high
A student performs this experiment but forgets to remove an air bubble from the N a O H buret valve. During the course of the titration, the air bubble did NOT disappear.
Would the measured volume of N a O H added in the back-titration be incorrectly high, incorrectly low, or will the volume remain unchanged?
- remains unchanged
- incorrectly low
- incorrectly high
remains unchanged
A student was performing this experiment with an antacid tablet which contained C a CO3 but forgot to heat the antacid solution prior to starting the back-titration. As sodium hydroxide was added the student noticed the formation of an insoluble white precipitate.
Would the presence of this precipitate cause the students experimentally determined volume of N a O H be incorrectly high or incorrectly low? Briefly explain.
Hint: Carbon dioxide reacts with water to form carbonic acid.
- Incorrectly low, the solution becomes less acidic if not boiled gently
- Incorrectly high, the solution becomes more acidic if not boiled gently
- Incorrectly low, the solution becomes more acidic if not boiled gently
- Incorrectly high, the solution becomes less acidic if not boiled gently
Incorrectly high, the solution becomes more acidic if not boiled gently
Use the following data to determine the percent mass of C a CO3 in an antacid tablet.
1.064 grams of antacid tablet dissolved
24.42 ml of 0.750 M HCl used to dissolved the tablet
4.02 mL of 1.00 M N a O H for the back-titration
To not type units with your answer.
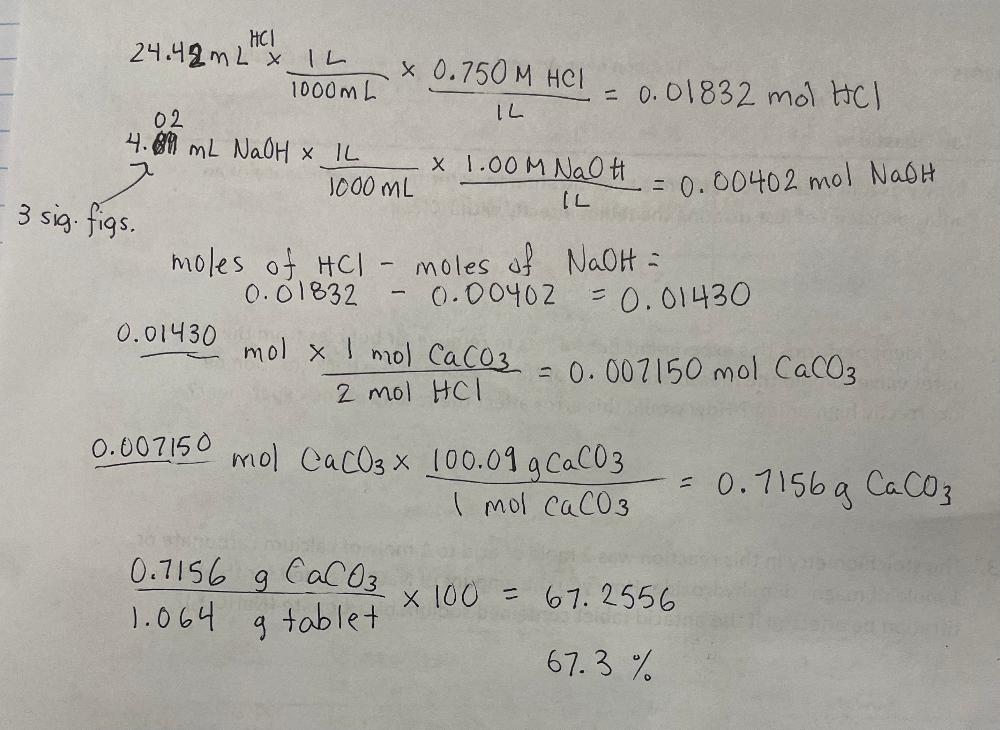
67.3