Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Aldehydes and Ketones
front 1 What is the product of oxidation of 2-butanone? | back 1 No reaction. |
front 2 Which compound has the lowest boiling point? A) CH3CH2CH2CH3 B) CH3CH2CH2OH C) CH3CH2CH3 D) CH3CH2OH E) CH3CH2CHO | back 2 C. |
front 3 What is the IUPAC name of Diisopropyl ketone? A) 2,4-dimethyl-3-propanone B) 2-dimethyl-3-pentanone C) 2,2-dimethyl-3-pentanone D) 2,4-dimethyl-3-pentanone | back 3 D. |
front 4 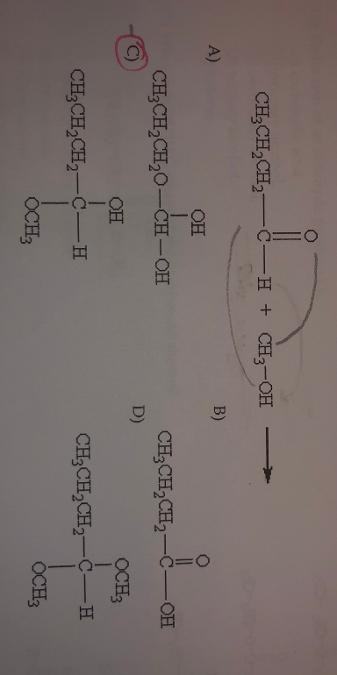 What is the product of the following reaction? | back 4 C. |
front 5 What type of compound does not contain a carbonyl group? A) aldehyde B) Amine C) Ketone D) ester E) Carboxylic acid | back 5 B. |
front 6 Which compound will give a positive Tollens’ Test? A) pentanoic acid B) pentane C) pentanal D) 2-pentanone E) 3-pentanone | back 6 C. |
front 7 Which of the following is not a property of aldehydes and ketones? A) They cannot form hydrogen bonds with water because they have no hydrogen Atoms bonded to oxygen. B) Most have distinctive odors. C) they are polar. D) they have higher boiling points than alkanes of similar molar mass. E) they have lower boiling points than alcohols of similar molar mass. | back 7 A. |
front 8 Hydrolysis of an acetal will produce: A) 2 aldehydes or ketones + one either B) 1 aldehyde or ketone + 2 alcohols C) 2 aldehydes or ketones + 1 alcohol D) 1 aldehyde or ketone + 2 waters E) 1 aldehyde or ketone + 2 ethers | back 8 B. |
front 9 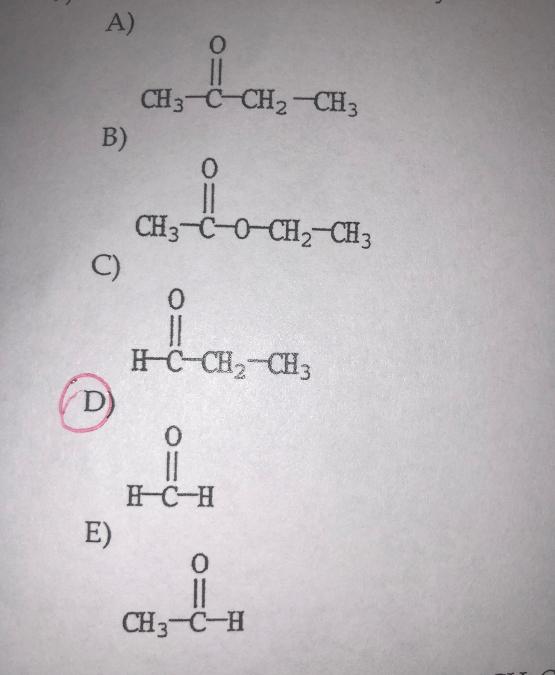 Which molecule is formaldehyde? | back 9 D. |
front 10 What is the correct name for: CH3CH(CH3)CH2COCH3 A) 4-methyl-2-butanone B) 4-methyl-2-pentanone C) 2-methyl-4-pentanone D) isobutyl acetone E) 2-methyl-4-butanone | back 10 B. |
front 11 What is the correct systematic name? CH3CH2COCH2CH2CH3 A) Ethyl methyl acetone B) Propyl methyl ketone C) 3-hexanone D) 4-hexanone E) methyl propyl ketone | back 11 C. |
front 12 Which compound has the highest boiling point? A) CH3COCH3 B) CH3CH2CHO C) CH3CH2OH D) CH3CH2CH2OH E) CH3CHO | back 12 D. |
front 13 What is the IUPAC name for acetone? A) 2-propanone B) 1-propanone C) dimethyl ketone D) 3-propanal E) 2-propanal | back 13 A. |
front 14 Reduction of an aldehyde produces a: A) carboxylic acid B) primary alcohol C) secondary alcohol D) tertiary alcohol E) ketone | back 14 B. |
front 15 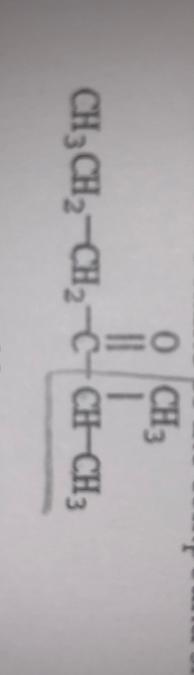 What is the IUPAC name of this compound? A) isopropyl n-propyl ketone B) 3-heptanone C) 4-heptanone D) 2-methyl-3-hexanone E) 5-methyl-4-hexanone | back 15 D. |
front 16 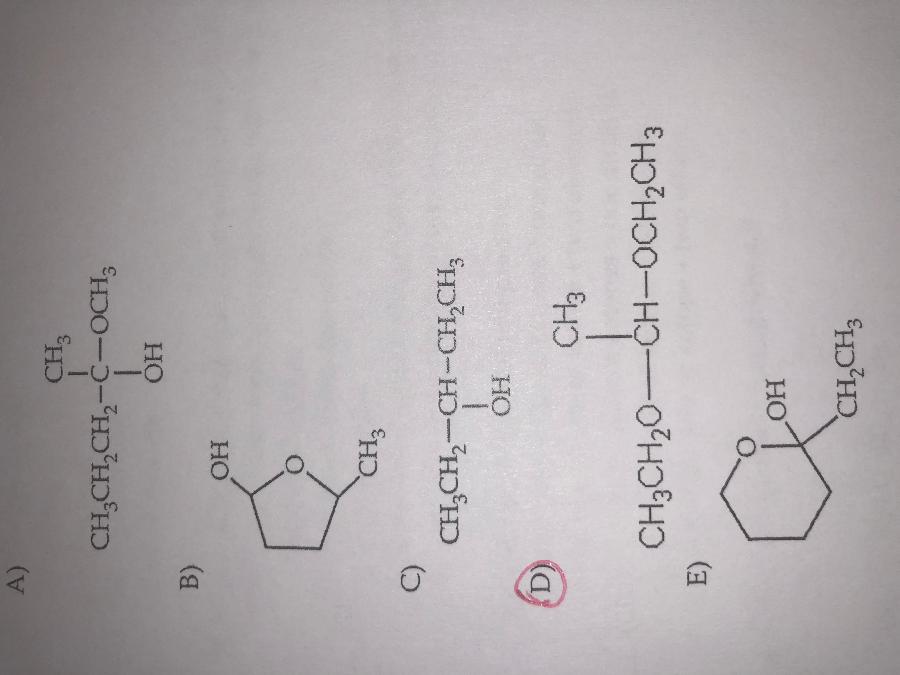 Which of the following is acetal? | back 16 D. |
front 17 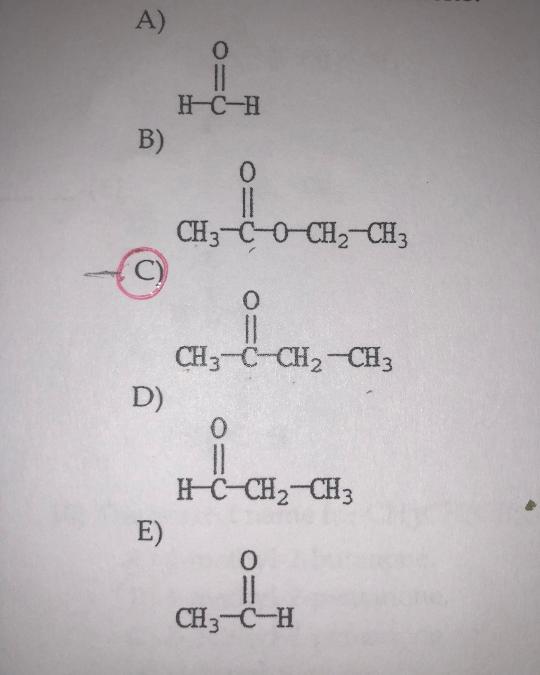 Which molecule is 2-butanone? | back 17 C. |
front 18 oxidation of a ketone produces: A) an aldehyde B) a carboxylic acid C) no reaction D) a secondary alcohol E) a primary alcohol | back 18 C. |
front 19 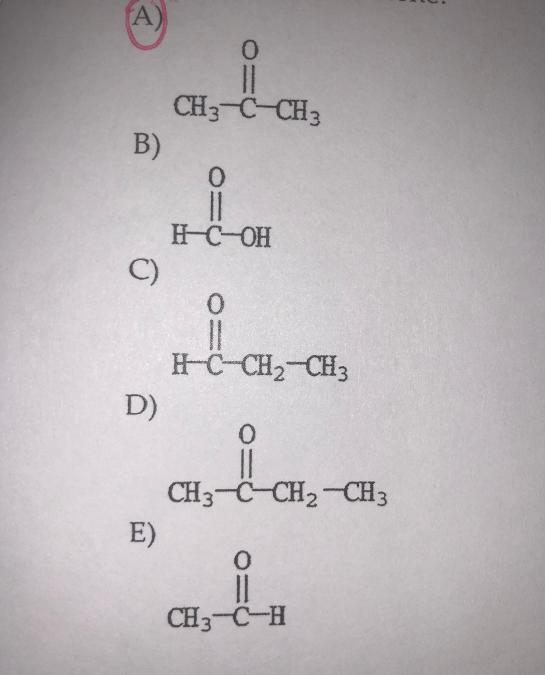 Which molecule is acetone? | back 19 A. |
front 20 Which observation denotes a positive Benedict’s test? A) A pale yellow solution with an older of chlorine changes to a purple color. B) hey purple solution yields a brown precipitate C) A red precipitate forms from a blue solution. D) A red-brown solution becomes clear and colorless. E) A mirror-like to posit forms from a colorless solution. | back 20 C. |