Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Micro Exam 2
front 1 a culture that contains a single species | back 1 pure culture |
front 2 cultures containing more than one type of microorganism | back 2 mixed culture |
front 3 procedures that (1) protect the culture and (2) protect you and the environment | back 3 aseptic/sterile techniques |
front 4 without contamination of yourself, others, the environment, the source culture, or the medium being inoculated | back 4 aseptic transfer |
front 5 free of living microbes (unable to reproduce) | back 5 sterile |
front 6 the process of something becoming impure or unclean | back 6 contamination |
front 7 a fluid culture medium used to grow microbes when fresh cultures or large numbers of cells are required; also used in microbial identification | back 7 broth |
front 8 used to grow stock cultures in a tube that can be refrigerated after incubation and maintained for several weeks | back 8 agar slants |
front 9 the bottom of this is used to measure the volume of a liquid | back 9 meniscus |
front 10 when must a microbiologist use a pure culture? | back 10 to isolate individual species from mixed culture so one can identify a specific organism |
front 11 what are the characteristics of a properly adjusted bunsen burner flame? | back 11 produces a flame with two cones (outer and inner), and the bottom knob adjusts the volume of gas (counter-clockwise increases the flame) |
front 12 sterilization of inoculating instruments is done in the hottest part of the flame - ______________________________, though heat-fixing bacterial smears on slides and incinerating the mouths of open glassware items may be done in _________________________ | back 12 the top of the inner cone the outer cone |
front 13 what are the instruments that microbiologists most often use to transfer microorganisms? | back 13 inoculating loops, inoculating needles, sterile pipettes are also used, and sterile swabs |
front 14 this pipette style is calibrated to deliver (TD) its volume by completely draining it and blowing out the last drop (the measurement markings end at the very end of the end of of the tip) | back 14 serological |
front 15 this style of pipette is not graduated, so fluid must be stopped at a calibration line; stopping the fluid beyond the last line on this pipette results in an unknown volume being dispensed (the measurement markings end before the very end of the tip) | back 15 Mohr pipette |
front 16 identify aseptic technique procedures that are performed before, during and after working with microorganisms | back 16 sterilizing the countertop, flaming the loop/needle, use the lid of the petri dish to contain airborne, alcohol-flame forceps and use the sterilized tips to transfer material, and disinfect the countertop again |
front 17 how are aerosols formed? | back 17 boiling droplets of microorganisms spray into the air when a loop or needle full of bacteria is thrust into a flame |
front 18 how do you prevent aerosols from forming? | back 18 flame loops and needles from the handle connection to the tip, so the bacteria are charred before incinerating them or wait for microbes to dry and flame sideways (slight angle, not completely sideways) |
front 19 explain how to protect yourself from laboratory-aquired infection | back 19 wear lab coat, closed-toed shoes, safety glasses, wash hands before leaving lab |
front 20 understand the purpose of stock cultures and why a new stock culture is always made as the first inoculating from a stock culture tube | back 20 we make a stock culture as a duplicate because the stock culture that we used for transfer is no longer sterile |
front 21 distinguish between prokaryotic and eukaryotic cells | back 21 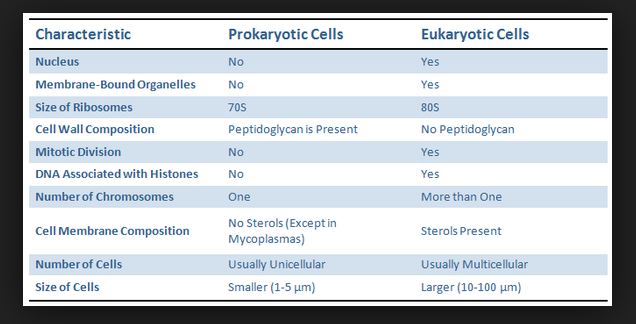 the biggest difference is that eukaryotes have membrane-bound organelles, while prokaryotes do not |
front 22 bacterial cells come in a variety of __________________ (shapes) and _____________________ (the number of planes in which division occurs and whether the cells separate after division) | back 22 morphologies arrangement |
front 23 Look over the morphologies (common shapes) and arrangements of prokaryotic cells on the microscope worksheet | back 23 good job |
front 24 the different morphologies of cells include (6 of them): | back 24 cocci (pl.) coccus (s.) - spheres bacilli (pl.) bacillus (s.) - rods spirlla (pl.) spirillum (s.) - spirals vibrios - slightly curved rods coccobacilli - short rods spirochetes - flexible spirals |
front 25  the cell arrangement prefix for pairs of cells is... and these two cell morphologies can form it | back 25  diplo- cocci and bacilli |
front 26  the cell arrangement prefix for chains of cells is... and these two cell morphologies can form it | back 26 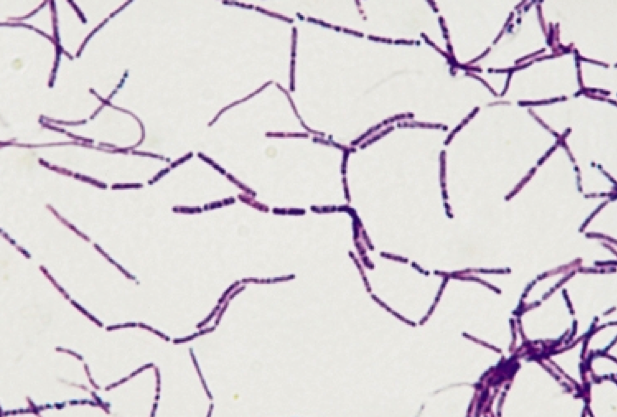 strepto- cocci and bacilli |
front 27 the cell arrangement for second division in a plane perpendicular to the first is called... and this arrangement is only formed with this cell morphology | back 27 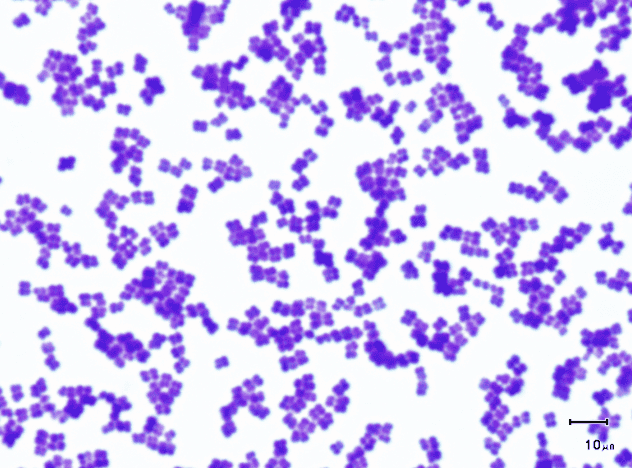 tetrad; cocci |
front 28 the cell arrangement for a third division plane perpendicular to the other two which produces a cube-shaped arrangement of eight cells is called... and this arrangement is only formed with this cell morphology | back 28 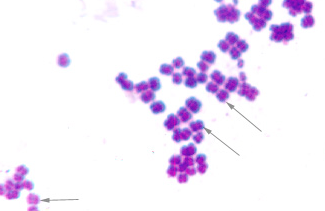 sarcina (cube); cocci |
front 29 the prefix is cell arrangement that has irregular division planes, or is a cluster of cells is called... and only this cell morphology can form it | back 29 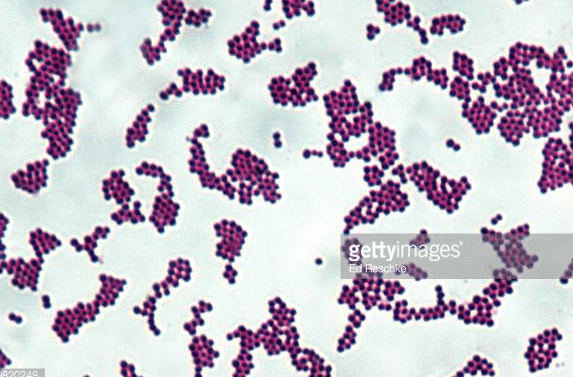 staphylo- cocci |
front 30 bacilli can also form __________ and ___________ cell arrangements | back 30 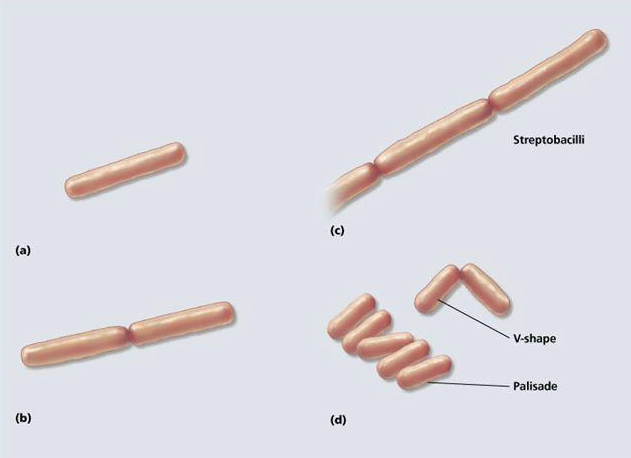 palisade and angular |
front 31 any bacterium of a genus that include the diphtheria bacillus, especially one that does not cause disease; a large group of nonpathogenic bacteria gram-positive bacteria that resemble corynebacterium | back 31 diphtheroids |
front 32 this arrangement is a stacking of rod-shaped cells side-by-side | back 32 palisade |
front 33 A state of "suspended animation" that some bacteria can adopt when conditions are not ideal for growth | back 33 spores |
front 34 differentiate spirilla and spirochetes by description and observation | back 34 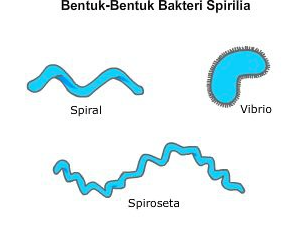 Both are spiral but spirilla are more rigid and use polar flagella for motility whereas spirochetes are flexible and motility through axial filaments. |
front 35 what is a vibrio? | back 35 slightly curved rods |
front 36  what does pleomorphic mean? | back 36  some bacterial species grow in a variety of shapes and are said to be pleomorphic |
front 37 differentiate heterotrophic eubacteria (bacteria) and cyanobacteria; recognize examples of each | back 37  bacteria are unicellular, microscopic and prokaryotic organisms; most
of them are devoid of chlorophyll |
front 38 __________ are thick walled spores; they have protective layers, like an endospore; It is usually formed after a heterocyst due to harsh environmental conditions | back 38 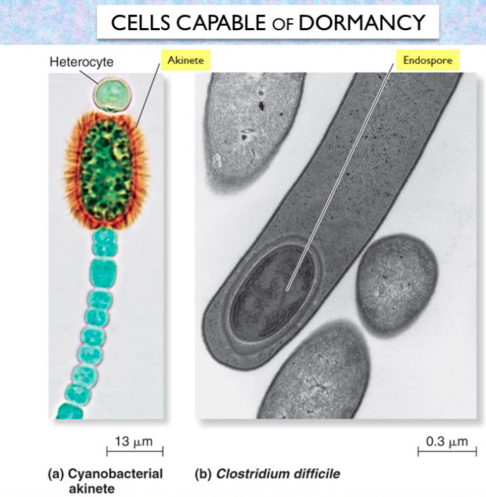 Akinete cyanobacteria |
front 39 this cell's sole function is to fix nitrogen when ammonia, nitrate, and other fixed forms of nitrogen are unavailable for a cyanobacteria | back 39 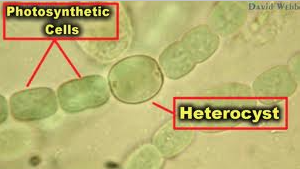 heterocyst cyanobacteria |
front 40 given the calibration factor of an ocular micrometer, determine the size of an object viewed through a microscope... at low power, each division in the ocular micrometer is ______ (include unit) at high power, each division in the ocular micrometer is ______ at oil, each division in the ocular micrometer is ______ | back 40 cell dimension = ocular units x calibration... low= 10 µm high= 2.5 µm oil= 1.0 µm |
front 41 this microscope consists of a single lens to enlarge an object | back 41 simple microscope |
front 42 this microscope includes an eyepiece and one or more objectives
where | back 42 compound microscope |
front 43 given the magnifications of an ocular and an objective lens, calculate the total magnification of a compound microscope | back 43 Total magnification= magnification by the objective lens X
magnification |
front 44 how much magnification is given by the ocular (eyepiece) lens? | back 44 10x |
front 45 magnification of lowest power is ______ plus ocular = total magnification of: _______ | back 45 4x 40x |
front 46 magnification of low power is ______ plus ocular = total magnification of: _______ | back 46 10x 100x |
front 47 magnification of high power is ______ plus ocular = total magnification of: _______ | back 47 40x 400x |
front 48 magnification of oil immersion is ______ plus ocular = total magnification of: _______ | back 48 100x 1000x |
front 49 explain how the use of immersion oil allows the microscopist to obtain greater resolution | back 49 Using immersion oil between the specimen and the lens increases the numerical aperture (measure of a len's ability to "capture" light coming from the specimen and use it to make the image) that makes its limit of resolution smaller. Oil causes a better resolution because the refractive index of oil is very similar to refractive index of glass; It increases better magnification by eliminating the transfer from glass to air |
front 50 describe the relationship between resolving power, numerical aperture, and the wavelength of light | back 50 Resolving power: actual measurement of how far apart two points must
be for |
front 51 Explain why a blue filter is used beneath the condenser of our microscopes (blue light is shorter wavelengths) | back 51 the blue filter increases contrast between a
specimen and its background; |
front 52 describe the change in working distance as magnification increases; what does this mean in practical terms with regard to observing samples at higher magnification? | back 52 As magnification increases, the working distance decreases (Working distance is the distance between the objective lens and the specimen) when observing samples at higher magnification, the microscopist has to be careful to not make the working distance completely squashed against the slide and lens |
front 53 evaluate the advantage to the microscopist of parfocality.. what does parfocal mean? | back 53 Parfocal: having corresponding focal points all in the same plane; having sets of objectives or eyepieces so mounted that they may be interchanged without varying the focus of the instrument (as a microscope) with which they are used Advantage: allows more accurate focusing at maximum focal length and
then |
front 54 **locate and name the parts of your microscope; explain the function of each part** | back 54 locate and explain the function |
front 55 list rules for the proper care of your microscope; know the checklist for putting a microscope away | back 55 -carry microscope to workstation using both hands (one hand grasping the microscope's handle and the other supporting the microscope beneath its base) -use lens paper to gently clean condenser and objective lens -when putting away, wipe the oil lens with lens paper and alcohol; lower stage, return objective lens to the 4x power; put bag back over and put back in storage spot |
front 56 describe the change in the size of the field of view as magnification increases; understand what that means in practical terms as one increases magnification when viewing a slide | back 56 As magnification increases size of field view decreases. The field of view is how much of your subject you see through the lens. One might not be able to see everything he/she needs to see with the higher power, so the mechanical stage would have to be moved to see different areas of specimen in the higher power |
front 57 recognize how the optics of the microscope change the orientation of the image seen when compared to the orientation on the slide | back 57 The optics of the microscope inverts the image (upside down); example was the "e" slide |
front 58 observe bacteria using oil immersion. Be able to identify using correct terminology the shape and arrangement of stained bacteria | back 58 okay |
front 59 compare the size of human blood cells with yeast cells and typical bacilli and cocci | back 59 Human blood cells ~12 um |
front 60 understand what is meant by the term "depth of field;" how was this observed in the cork and threads' fibers slides? | back 60 - distance through which you can move the specimen and still have it
remain in focus |
front 61 With compound light microscope we are able to see objects as small as | back 61 0.1 micometer (um) or 100 nanometers (nm) |
front 62 Working distance is | back 62 the distance between the objective lens and specimen |
front 63 As working force decreases the magnification | back 63 increases |
front 64 when the stage moves to the right, the image moves to the... | back 64 left |
front 65 when the stage moves toward you, the image moves... | back 65 away from you |
front 66 Why aren't the magnifications of both ocular lenses of binocular microscope used to calculate total magnification? | back 66 Because the image only goes through one ocular to reach both eyes |
front 67 Assuming that all other variables remain constant, explain why light of shorter wavelengths will produce a clearer image that light of longer wavelengths | back 67 calculate for limit of resolution D = wavelength/(NA object + NA condensor) visible light = 300-700 nm limited resolution decreased if wavelength decreased |
front 68 why is wavelength the main limiting factor on limit of resolution in light microscopy? | back 68 if wavelengths are small = smaller limit resolution |
front 69 Why should closing the iris diaphragm improve your ability to determine thread order? | back 69 by closing the iris diaphragm, it closes the peripheral light giving it a better image |
front 70 what does the colored-threads slide demonstrate about specimens you will be observing later in the class? | back 70 even though the specimens may be very small, they still have depth |
front 71 list two common uses for poured plates | back 71 -for streak plates (isolation of microbes to identify and create a pure culture) -for spread plates (to create a uniform lawn) -often used for isolating individual species from a mixed culture -counting the number of cells in a diluted sample |
front 72 understand the process of preparing and storing stock cultures | back 72 Prepare new stocks by taking sample from old stock about to be used. Fish tail spread onto new slant using sterile inoculating loop and store at 25°-35°C depending on organism to be grown. Do not tighten cap all the way. |
front 73 describe and practice the process of preparing sterile plates | back 73 sterile plates are done by using sterile agar broth, flamed at the mouth of the tube right after opening, poured with the petri plate lid only slightly open and quickly closed, flame the empty tube and screw back on the lid |
front 74 understand why, when preparing plated media, it would be useful to incubate the plates before using them | back 74 if the plate has been refrigerated this will prevent cold sensitive bacteria from shock or death; will evaporate any excess condensation on the media make sure they are sterilized and have no unwanted growth before growing microbes |
front 75 identify common sources of contaminants when preparing plated media | back 75 air, lip of tube |
front 76 distinguish between a mixed culture and a pure culture | back 76 A mixed culture contains two or more species of microbe; |
front 77 explain why a microbiologist would need pure cultures | back 77 the identification process of an unknown microbe relies on obtaining a pure culture of that organism the streak plate method produces individual colonies on an agar plate, then a portion of an isolated colony then may be transferred to a sterile medium to start a pure culture |
front 78 understand why plates are labeled on the bottom of the dish and inverted for incubation and storage | back 78 to avoid condensation disturbance of growth as well as rehydrate bacterial colonies |
front 79 decreasing quantity | back 79 concentration gradient |
front 80 growth of culture over entire surface of media (individual colonies are indistinguishable) | back 80 confluent growth |
front 81 method in streak plating to obtain a pure culture. The petri dish is labeled on the bottom in four quadrants (0, I, II, III). Initial inoculum is spread in 0 quadrant. Each following spread will be taken from previous quadrant after sterilization of inoculating instrument, and each successive quadrant should exhibit less dense microbial growth with isolated colonies present in quadrant III and possibly II | back 81 quadrant method |
front 82 sample being introduced to sterile culture media | back 82 inoculum |
front 83 tube with solidified agar perpendicular to opening, as opposed to the slant. Used to study the gas requirements of microorganisms (aerobic vs anaerobic) | back 83 agar deep |
front 84 a macroscopically visible cluster of identical microorganisms | back 84 colony |
front 85 this encompasses colonies that may arise from an individual cell, a pair of cells, chains, or clusters | back 85 colony forming unit (CFU) |
front 86 give the goal for preparing a streak plate and describe the method for preparing a plate | back 86 sterilized Inoculation loop used to drag bacterial sample through 4 quadrants (0,1,2,3) for the purpose of isolating individual colonies or pure cultures |
front 87 Give the goal for preparing spread plates and describe how they are made | back 87 Swab used to create a uniform lawn of bacteria for the purpose of seeing response to certain treatments |
front 88 Prepare streak plates and spread plates, avoiding common pitfalls and contamination. (Note: The streak plate method used in this laboratory is provided on Bb in the Supplement (Ex 13). | back 88 Grading rubric involves |
front 89 understand the assumptions and limitations of these procedures | back 89 Assumption is that colonies can be isolated or even lawn can be made.
|
front 90 Identify use(s) for streak plates and spread plates | back 90 streak plates: a method of bacterial isolation spread plates: used for antibiotic and disinfectant testing |
front 91 Understand factors that will affect the size of colonies on plates | back 91 technique, nutrients, waste products, and antibiotics could all cause smaller colony formation |
front 92 understand the purpose for using agar in biological media | back 92 it is a gel at room temperature and most microbial species
cannot break it down for food. It provides a growing matrix
for microbial species through microscopic channels liquid filled
channels that allow nutrients to diffuse through as food for
microbes |
front 93 Describe how the probable size of an inoculum will affect the way in which a streak plate will be prepared. (eg. size of the quadrants, number of “dips” into the previous quadrant.) | back 93 Large inoculum would need larger quadrants and less dips into previous quad to spread out colonies more |
front 94 this is a measure of how cloudy or hazy a solution is as a result of bacterial growth | back 94 turbidity |
front 95 what does the bacterial solution we made get compared to? | back 95 the McFarland standard |
front 96 Why do we need to standardize the inoculum on a spread plate? Why don't we do this for a quadrant streak? | back 96 spread plates must be consistent to form a proper bacterial lawn to be used for testing; a known and consistent concentration of bacteria is spread on all plates we do not standardize quadrant streaks because consistency is not the goal, isolation is the goal. |
front 97 List four characteristics of microbial morphology as identified in the lab book | back 97 1. color |
front 98 positively charged ion | back 98 cation |
front 99 negatively charged ion | back 99 anion |
front 100 stains are solution consisting of a __________ (usually water or ethanol) and a colored molecule (often a benzene derivative), the ___________ | back 100 solvent chromogen |
front 101 the portion of the chromogen that gives it its color | back 101 chromophore |
front 102 the charged portion of a chromogen that allows it to act as a dye through ionic or covalent bonds between the chromogen and the cell | back 102 auxochrome |
front 103 differentiate between basic and acidic dyes and understand how they work. | back 103 An acidic dye carries a negative charge because it
is missing hydrogen; leaves the cell negatively charged and stains the
background, repelling the bacteria |
front 104 Understand why, when using solid media, the inoculum is serially diluted on the slide | back 104 the concentration of the bacteria is too high, so serial dilution takes place to help isolate the bacterias on the slide better. |
front 105 Know the purpose of air-drying (or gently warming) smears | back 105 excess water left on the slide will boil during the fixing stage, causing most microbes present to rupture and become airborne |
front 106 Know the purpose and effects of heat fixing a slide | back 106 Basic stains are heat fixed. Heat fixing kills bacteria, makes them adhere to the slide, and coagulates cytoplasmic proteins to make them more visible under the microscope |
front 107 What are some consequences of leaving a stain on a bacterial smear too long (over-staining)? | back 107 this can make the cell appear larger than it really is, with a simple stain it might be okay but with gram staining it would ruin it Overstaining the bacterial smear may cause the cell wall disruption or totally destroy the cell wall which results in the loss of true morphological characteristics of the bacterial cell |
front 108 What are some consequences of not leaving a stain on a bacterial smear long enough (under-staining)? | back 108 The cells may lose the stain when washed with alcohol or water which
causes a problem in identifying the cell |
front 109 Consider a coccus and a rod of equal volume, which is more likely to survive in a dry environment? | back 109 Cocci, with their low surface to volume ratio are less efficient at exchange with the environment than rods, but are at an advantage in a dry environment where they lose water dehydrate more slowly than rods |
front 110 Consider a coccus and rod of equal volume, which is more likely to survive in a moist environment? | back 110 Organisms with high surface to volume ratio (rods,spirilla) often survive better in moist environments where their ability to exchange materials with their surroundings is an asset for nutrient acquisition of water loss is not a concern |
front 111 Identify two common negative stains: | back 111 Congo red and Nigrosin (India Ink) |
front 112 Explain how a negative stain interacts with bacteria and the result obtained | back 112 the negative stain uses a dye solution in which the chromogen is
acidic and carries a negative charge |
front 113 Understand why slides prepared with negative stains are not heat fixed | back 113 negative stains cannot be heat fixed because they are too delicate to withstand heat-fixing; the morphology would be destroyed and size would shrink immensely |
front 114 Understand the meaning of the rule: “draw the medium, do not push it” when making negative stained slides | back 114 -Gently draw a second slide across the surface of the first until it
contacts the drop so that the drop will spread across the edge of the
top slide |
front 115 Identify two reasons for using a negative stain | back 115 -used to determine morphology and cellular arrangement in bacteria that are too delicate to withstand heat-fixing -to avoid distorting of the cell |
front 116 there are composed of mucoid polysaccharides or polypeptides that repel most stains because of their neutral charge | back 116 capsules |
front 117 Know what a bacterial capsule is and how to demonstrate its presence by staining | back 117  Excreted by cell to form clear, gelatinous, protective layer Background will appear darker (negative stain), then a clear barrier surrounding the stained cell |
front 118 why doesn't a negative stain colorize the cells in the smear? | back 118 the negative stain doesn't colorize the cells in the smear because the negative stain's chromogen, which carries a negative charge, is repelled by the negative charge on the cell's surface therefore the stain colors only the background, allowing visibility of the cell and/or its capsule |
front 119 Eosin is a red stain and methylene blue is blue. What should be the result of staining a bacterial smear with a misture of eosin and methylene blue? | back 119 Eosin--is acidic and acts as a negative stain The smears background would turn out red while the cells would turn out blue. |
front 120 Capsules are neutrally charged. this being the case, what is the purpose of emulsifying the sample in serum in this staining procedure? | back 120 serum acts as glue to hold it to the slides |
front 121 Some oral bacteria produce an extracellular "capsule." Of what benefit is a capsule to these cells? | back 121 capsules help bacteria stick to teeth internally, capsules help prevent phagocytosis |
front 122 Summarize the history and importance of the Gram stain | back 122 The Gram stain was developed in 1884 by Hans Christian Gram. It is one of the most useful staining procedures because it classifies bacteria into two large groups: gram positive and gram negative |
front 123 Identify the primary stain, mordant, decolorizer and counterstain used in the Gram staining procedure | back 123 primary stain: crystal violet |
front 124 Identify the purpose of each reagent of the Gram staining procedure | back 124 The crystal violet stains all the cells The iodine forms a crystal violet-iodine complex with the crystal violet to keep it in the cell, called a mordant The decolorizer (alcohol or acetone) allows the crystal violet-iodine complex to leave the gram-negative cells The counterstain (Safranin) stains the gram-negative cells pinkish red |
front 125 Describe and compare cell wall structure of gram-positive and gram-negative organisms | back 125 Gram negative cell walls have a higher lipid content (because of the outer membrane) and a thinker peptidoglycan later than Gram-positive cell walls the alcohol/acetone extracts the lipid, making the gram-negative wall more porous and incapable of retaining the crystal violet-iodine complex, thereby decolorizing itt the thicker peptidoglycan and greater degree of cross-linking (because of techoic acids) trap the crystal violet-iodine complex more effectively, making the Gram-positive wall less susceptible to decolorization |
front 126 Understand how the differing wall structures are affected by the decolorization step of the Gram stain | back 126 the alcohol/acetone extracts the lipid, making the gram-negative wall more porous and incapable of retaining the crystal violet-iodine complex, thereby decolorizing it the thicker peptidoglycan and greater degree of cross-linking (because of techoic acids) trap the crystal violet-iodine complex more effectively, making the Gram-positive wall less susceptible to decolorization |
front 127 Identify the characteristics gram-nonreactive bacteria and those bacteria for whom the Gram stain is not appropriate; give examples | back 127 Gram-nonreactive bacteria are bacteria without a cell wall such as Mycoplasma species, or with cell walls resistant to this staining procedure such as Mycobacterium species Cultures 24 hours old or less should be used, or else they will lose their ability to retain the crystal violet-iodine complex |
front 128 Identify the variables which can affect the results of a Gram stain and understand how to limit the effects of these variables | back 128 The stain can be over-decolorized by leaving the alcohol on too long, creating reddish gram-positive cells The stain can also be under-decolorized, creating purple gram-negative cells Inconsistency in preparation of the emulsification can also occur |
front 129 predict the effect on Gram-positive and Gram-negative cells of the following "mistakes" made when performing a Gram stain. consider each mistake independently 1) failure to add the iodine | back 129 The Crystal Violet would be leeched out of everything, because the Iodine is what cross-links with the Crystal Violet. And that cross-link is what help the stain to stay within the cell during decolorization. When the counterstain, Safranin, is applied everything will end up pink and appear Gram-negative. |
front 130 predict the effect on Gram-positive and Gram-negative cells of the following "mistakes" made when performing a Gram stain. consider each mistake independently 1) failure to apply the decolorizer | back 130 Everything will look purple at the end of the staining procedure |
front 131 predict the effect on Gram-positive and Gram-negative cells of the following "mistakes" made when performing a Gram stain. consider each mistake independently 1) failure to apply the safranin | back 131 You would not be able to see the Gram-negative cells. The Gram-positive would be the only cells that are colored and therefore visible. Gram-negative would be clear and therefore not visible |
front 132 predict the effect on Gram-positive and Gram-negative cells of the following "mistakes" made when performing a Gram stain. consider each mistake independently 1) reversal of crystal violet and safranin stains | back 132 a) Everything would appear purple at the end. |
front 133 Both Crystal Violet and Safranin are basic stains and may be used to do simple stains on gram positive and gram-negative cells. This being the case, explain how they stain different cell types in the Gram stain. | back 133 a) The properties of the stains themselves, the Crystal Violet will
cross-link with the mordant, Gram Iodine and the Safranin will not
cross-link. |
front 134 If you saw large eukaryotic cells in the preparation made from your
gumline, they were most likely your own epithelial cells. | back 134 No, only Bacteria are Gram-positive or Gram-negative. |
front 135 If you saw large eukaryotic cells in the preparation made from your
gumline, they were most likely your own epithelial cells. | back 135 Human cells do not have cell walls or Peptidoglycan (PDG). The cells could take either color stain. |
front 136 One of your lab partners has followed the recommended procedure of running Gram-positive and Gram-negative control organisms on her Gram stain of an unknown species. Her choices of controls were Escherichia coli (gram-negative) and Bacillus subtilis (gram-positive). She tries several times and each time concludes she is decolorizing too long because both controls have pink cells, one more than the other. What might you suggest she try and why? | back 136 I would suggest that she make sure she is using new cultures, because if she is using old cultures then they lose their ability to retain the Crystal Violet. |
front 137 what makes acid-fast organisms different from others? | back 137 they have mycolic acid in the cell walls of the organisms; this is the cytological basis for the acid-fast differential stain |
front 138 what is the mycolic acid? | back 138 it is waxy substance that gives acid-fast cells a higher affinity (bond with) for the primary stain (carbolfuchsin) and resistance to decolorization by an acid alcohol solution |
front 139 Prepare mixtures of acid-fast and non-acid fast bacteria, stain them with the Kinyoun or Ziehl-Neelsen procedure, and learn to interpret the results accurately. | back 139 Both: primary stain for 5 minutes, decolorize, counter stain for 1
minute |
front 140 Identify genera that include acid-fast species | back 140  Mycobacterium, Nocardia, Cryptosporidium, Isospora |
front 141 List the reagents utilized in the Kinyoun and Ziehl-Neelsen procedures and identify the purpose of each reagent. | back 141 ZN and K: the only difference is that ZN uses heat-fixing (steam), K method also uses a slightly more lipid-soluble and concentrated carbolfuchsin; brilliant green or methylene blue can be used as the counterstain |
front 142 Evaluate differences between the Kinyoun and Ziehl-Neelsen methods of acid-fast staining. | back 142 Z uses steam to stain, K uses a more lipid-soluble carbolfuchsin stain |
front 143 Identify the composition of the decolorizer. Explain why acid-fast bacteria are not decolorized | back 143 95% ethanol, 3% HCl the mycolic acid in their cell walls resist the decolorization; the primary stain carbolfuchsin is lipid soluble and penetrates the waxy cell wall |
front 144 Understand why acid-fast bacteria are considered Gram nonreactive | back 144 their waxy cell walls repel typical aqueous stains |
front 145 Explain the role mycolic acids theoretically play in the acid-fast staining reaction | back 145 give the cell a higher affinity (liking) for the stain and help it resist decolorization |
front 146 Understand the reason for using a drop of serum to prepare the smear for the acid fast stain | back 146 to help the "slippery" acid-fast cells adhere to the slide |
front 147 How does heating the bacterial smear during a ZN stain promote entry of carbolfuchsin into the acid-fast cell wall? | back 147 help drive the primary stain into the waxy cell walls of these difficult-to-stain cells |
front 148 Are acid-fast negative cells stained by carbolfuchsin? If so, how can this be a differential stain? | back 148 Yes, they are initially stained by carbolfuchsin but the decolorizing step, acid alcohol, removes stain from acid fast negative cells while acid fast positive cells retain the stain |
front 149 Why do you suppose the acid-fast stain is not as widely used as the Gram stain? When is it more useful than the Gram stain? | back 149 Acid fastness is a characteristic that is shared by just a few organisms. Many bacterial cells are easily stained with simple stains or using the Gram stain. -acid fast is useful when acid fast positive bacteria are suspected |
front 150 Prepare a mixture of spore forming bacteria and non-spore forming cocci , stain these slides using the Schaeffer-Fulton procedure and learn to interpret the results accurately. | back 150 1. Begin with a heat-fixed emulsion |
front 151 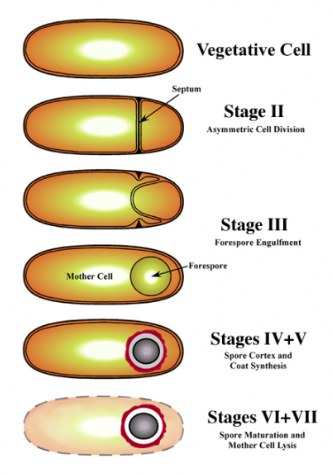 Prepare smears of various spore-forming bacteria and stain these using the Schaeffer-Fulton procedure and learn to interpret the results accurately. | back 151 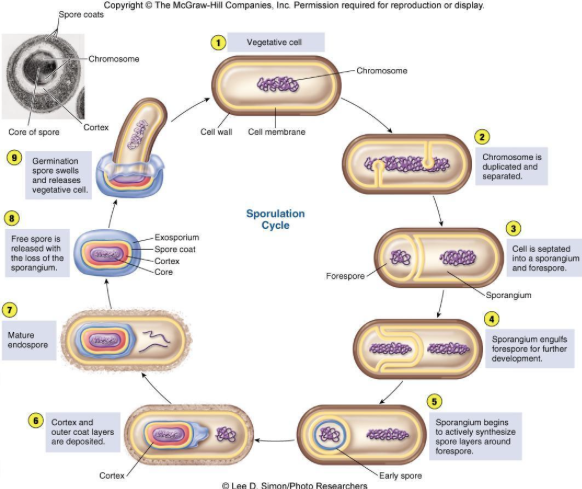 ta-da |
front 152 Know and understand the purpose of each step in the Schaeffer-Fulton endospore staining procedure. (Be able to identify the decolorizer.) | back 152 1. all cells are stained with Malachite green using steam to force
the stain into the spore, if present |
front 153  Identify the location of spores within the sporangium. Be able to identify terminal, subterminal and central spores. | back 153 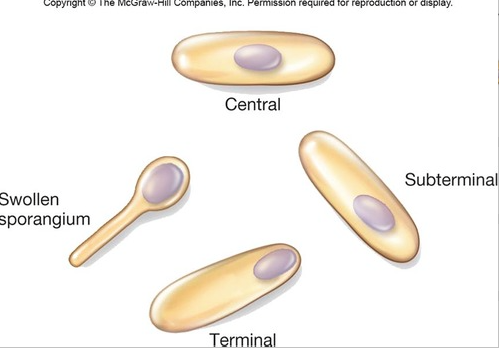 terminal: at the end of the cell |
front 154 a state in which there is no metabolic activity | back 154 cyptobiotic state |
front 155 where spores are produced | back 155 sporangium |
front 156 the protein that makes up the tough outer covering of the spore | back 156 keratin |
front 157 responsible for producing and housing the endospore | back 157 spore mother cell |
front 158 Identify three characteristics of spore formation used by microbiologists to help identify bacterial spore formers. | back 158 location in cell |
front 159 Identify two the bacterial genera that form endospores. Identify the one known spore forming coccal genera. | back 159 Bacillus and Clostridium Sporosarcina |
front 160 List two endospore forming genera which also produce antibiotics. | back 160 1) endospore-forming rod that causes diarrhea and pseudomembranous colitis. This organism is the leading cause of antibiotic-associated diarrhea (AAD). This organism's nickname is "C-diff." ..... Clostridium difficile |
front 161 why does this exercise call for an older 5-day culture of bacillus? | back 161 Young cultures of spore-forming microbes may not demonstrate any endospores because the vegetative cells may not have been subjected to sufficient stress to stimulate sporulation |
front 162 What does a positive result for the spore stain indicate about organisms? what does a negative result for the spore stain indicate about the organism? | back 162 positive results: shows cell has ability to produce spores, which
narrows down indentification of bacteria to a few genera of bacteria |
front 163 why is it not necessary to indicate a negative control for this stain procedure? | back 163 Endospore stain is a differential staining procedure that allows to see both spores and vegetative cells, thus including separate -ve control consisting of only vegetative cells is not required |
front 164 Endospores do not stain easily. Perhaps you have seen them as unstained white objects inside Bacillus species in other staining procedures. If they are visible as unstained objects in other stains, of what use is the endospores stain? | back 164 unstained objects can be something other than an endospore, such as lipid granules |
front 165 a series of controlled transfers down a line of dilution blanks. the series begins with a sample containing an unknown concentration of cells (density) and ends with a very dilute mixture containing only a few cells | back 165 serial dilution |
front 166 agar is solid in tube (leveled) and culture is stabbed into agar | back 166 agar pour (deep) |
front 167 TNTC | back 167 too numerous to count |
front 168 TFTC | back 168 too few to count |
front 169 CP | back 169 countable plate |
front 170 to impress lenticules on a surface or film | back 170 lenticulate |
front 171 cell or group of cells that produces a colony when transferring to a plated media | back 171 colony forming unit (CFU) |
front 172 can be used to determine the number of viable bacteria in a food sample | back 172 heterotrophic plate count |
front 173 growth medium to assess or monitor bacteria growth of a sample | back 173 standard methods agar aka plate count agar |
front 174 Differentiate a single cell from a colony-forming unit | back 174 a colony-forming unit (CFU) can be any number of cells, whether a single cell or a group of two or more cells; a single cell is only one single cell |
front 175 Understand the process of serial dilution and how one calculates the dilution factor for each dilution made | back 175 When 0.1 mL of a 10-4 solution is transferred to a plate,
what is the volume of the sample in the plate? |
front 176 Define a countable plate and understand why plates with fewer and greater numbers of colonies are unreliable | back 176 <30 colonies, statistically unreliable TFTC |
front 177 Understand the basic assumption made concerning the number of bacteria in the inoculum and the number of colonies on a plate | back 177 the number of colonies is equal to the number of bacteria per gram of meat transferred |
front 178 Understand the formulas used to determine the concentration of bacteria in the original sample after making plate counts from a dilution series (serial dilution) | back 178 x colonies _________ 1x10- x dilution so...... (x colonies)(1x10x) |
front 179 Give reasons a microbiologist (or the health department) might wish to ascertain the number of bacteria per milliliter in a sample of meat. | back 179 -for the Health and Safety tests (must be under 106 bacteria/ gram of meat) -Medical microbiologist manipulate population density for use for standardized testing -To measure the effect of varying nutritional or environmental conditions. -Industrial microbiologist maintain populations at optimum levels in products including enzymes, antibiotics, beer and wine |
front 180 Know the maximum number of bacteria per gram of meat for the meat to be saleable | back 180 must be under 106 bacteria/ gram of meat |
front 181 Understand the limitations of the procedures used to estimate bacterial numbers | back 181 -one limitation is that only bacteria capable of growing in the culture medium under the environmental conditions provided will be counted |
front 182 You were instructed to add 1.0 mL out of 5.0 mL of an undiluted sample to 99 mL of sterile diluent. Instead,you add all 5.0ml to the 99 mL. What was the intended dilution and what is the actual dilution? | back 182 intended= 1x10-2
|
front 183 a nutrient agar plate labeled 10-5 mL produced 154 colonies after incubation what was the cell density in the original sample? what combination(s) of volumes and dilution factors could have been used to inoculate this plate? | back 183 The original cell density (OCD) = 1.54 x 107 CFU/mL 1 mL was used to inoculate this plate |
front 184 a nutrient agar plate labeled 10-7 mL produced 62 colonies after incubation what was the cell density in the original sample? what combination(s) of volumes and dilution factors could have been used to inoculate this plate? | back 184 The original cell density (OCD) = 6.2 x 108 CFU/mL 1 mL was used to inoculate this plate |
front 185 why is a standard plate count performed on food products? | back 185 to determine number of microbes in 1g of food if greater than 106 it may be hazardous to consume |
front 186 Distinguish between transient and resident microbial flora | back 186 transient - temporary microorganisms that can be washed off hands with soap or other detergent resident - long-term flora that generally inhabit hair follicles and sebaceous glands of skin that remain even after thoroughly washing, scrubbing, and disinfecting hands |
front 187 Describe how soap and detergent differ | back 187 Detergent is any cleansing solution, including soap, to remove bacteria embedded in fats and oils Soap is formed when fats are heated in the presence of a name such as sodium hydroxide (NaOH) They both decrease surface tension so that microbes can be more easily removed |
front 188 Explain how soap cleans, especially with regard to oily or greasy substances | back 188 In greasy water, the hydrocarbon end of the soap molecule is soluble in oil and the carboxylate end is soluble in water. A chemical link forms between oil and water. Then the oily ends of the molecules dissolve together. Now the greasy oil is chemically combined with water; it can be carried away in the liquid. |
front 189 Understand why soap is not an effective cleansing agent when used with hard water (such as Jacksonville water). | back 189 Hard water is water containing mineral salts. When soap is used with hard water, magnesium and calcium salts react with soapy oil droplets, forming a lighter than water precipitate, scum. This scum, with its load of grease and bacteria, adheres to whatever is being washed. |
front 190 Evaluate the effectiveness of an antiseptic detergent in removing surface organisms | back 190 Antiseptic detergents work better than regular soap |
front 191 Describe the importance of preparing control plates when performing experiments like this one | back 191 It's important to have control plates for this experiment so you can distinguish between bacteria that possibly came from the substance/object you were using and the resident/transient bacteria on the hand. |
front 192 understand why the single line with zigzag was used to separate the organisms (CFU=s) for this experiment | back 192 It was an attempt to spread out the possible different bacteria on the hand |
front 193 Praise Jesus | back 193 yippee! |