Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
CHEM115 midterm reveiw3
front 1 How many grams of oxygen (O2) are needed to completely react with 24.0 g of propane (C3H8) according to the equation below? | back 1 a. 0.545 g b. 2.73 g c. 87.2 g d. 28.8 g |
front 2 Catalysts lower the activation energy of a chemical reaction. | back 2 True False |
front 3 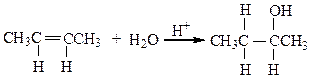 The reaction below is an example of a ___ reaction. | back 3 a. hydrogenation b. hydrolysis c. dehydration d. hydration |
front 4 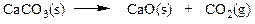 Classify the reaction below as involving synthesis, decomposition, single replacement, or double replacement. | back 4 a. single replacement b. double replacement c. decomposition d. synthesis |
front 5  Which set of coefficients properly balance the equation below, going from reactants to products? | back 5 a. 1,3,2,3 b. 1,7,4,3 c. 2,7,4,6 d. 2,3,2,3 |
front 6 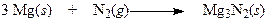 Regarding the following reaction, | back 6 a. This reaction does not involve oxidation or reduction. b. Magnesium is oxidized and nitrogen is reduced. c. Both magnesium and nitrogen are reduced. d. Magnesium is reduced and nitrogen is oxidized. |
front 7 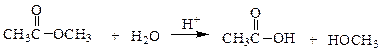 In the following reaction, how many grams of water are needed to completely hydrolyze 30.8 g of the ester? | back 7 a. 7.48 g b. 0.416 g c. 30.8 g d. 11.3 g |
front 8 What was the earliest reported method of fighting infection in the operating room? | back 8 a. The patient was treated with Betadine before surgery. b. Carbolic acid was used on the surgeon’s tools. c. Listerine was the antiseptic of choice. d. The surgeons washed with soap and chlorine water. |
front 9 Ethene, C2H4, burns in air according to the equation below. What is the theoretical yield (in grams) of CO2 when 3.24 g of C2H4 are reacted with 4.83 g of O2? | back 9 a. 3.24 g b. 9.72 g c. 0.151 g d. 4.43 g |
front 10 An oxidation reaction can be defined as when an atom loses an oxygen. | back 10 True False |
front 11 2-methylcyclopentene reacts with hydrogen in the presence of platinum catalyst to form methylcyclopentane. In this reaction: | back 11 a. 2-methylcyclopentene is reduced b. Pt is oxidized c. methylcyclopentane is reduced d. cyclohexane is oxidized |
front 12 The function of an enzyme in a reaction is to | back 12 a. cause a reaction to be reversible at the same reaction rate. b. increase the ∆G to increase the rate of reaction. c. increase the rate of reaction by reducing the activation energy. d. inhibit the reaction. |
front 13 The subscripts of a chemical formula can be changed to balance a chemical equation. | back 13 True False |
front 14 Benzoyl peroxide can be used as a treatment for acne. Benzoyl peroxide | back 14 a. has a reduction action that tends to neutralize the effects of the acne bacteria. b. is injected so that it will have a general immune action against bacteria causing acne. c. is an oxidizing agent used on the skin to kill the bacteria causing acne. d. is not used for acne; it is an antiseptic used to sterilize a surgeon’s hands. |
front 15 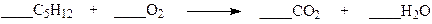 Which set of coefficients properly balance the equation below? | back 15 a. 1,1,5,6 b. 1,5,5,6 c. 1,5,5,1 d. 1,8,5,6 |
front 16 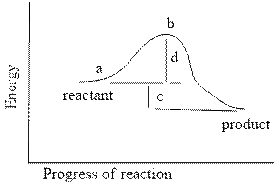 In the reaction energy diagram below, the activation energy is represented by: | back 16 a. c b. a c. d d. c |
front 17 What mass of potassium chloride, a salt substitute often used by heart patients, can be produced directly from 5.2 g potassium and 7.9 g chlorine? | back 17 a. 5.2 g KCl b. 4.9 g KCl c. 9.9 g KCl d. 10.1 g KCl |
front 18 Which reaction is NOT an oxidation-reduction reaction? | back 18 a. b. c. d. All of these are oxidation-reduction reactions. |
front 19 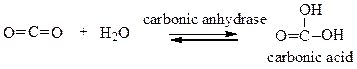 Choose the comment that is appropriate for the reaction: | back 19 a. Carbonic anhydrase catalyzes the conversion of carbon dioxide into carbonic acid. b. The reaction runs in forward direction only. c. This reaction runs in backward direction only. d. This is a reaction that only occurs in the laboratory. |
front 20 Methanol burns in air according to the equation below. How many grams of methanol can be burned by 10 grams of oxygen? | back 20 a. 2 g b. 7 g c. 10 g d. 20 g |
front 21 When the pressure is increased above 760 torr, the boiling point of water | back 21 a. decreases to temperatures below 100 C. b. remains the same at 100C. c. increases to temperatures above. d. drops to temperatures below 0C. |
front 22 Ethyl alcohol (CH3CH2OH) is completely soluble in water. It is made of both polar and non-polar groups. Ethyl alcohol is best classified as a(n) ___ substance. | back 22 a. hydrophilic b. hydrophobic c. lipophilic d. amphipathic |
front 23 What is the volume of a mole of an ideal gas under standard conditions (STP)? | back 23 a. 0.0821 liter b. 760 liters c. 22.4 liters d. 1.0 liter |
front 24 The gas constant is used in some calculations. The value of the gas constant is | back 24 a. to make certain that all gases are ideal gases. b. 0.0821 L atm/mol K. c. 25°C and 1 atm. d. 0°C and 1 atm at 1-L volume. |
front 25 Identify the compound that is insoluble in water. | back 25 a. Ba3(PO4)2 b. Ba(NO3)2 c. K3PO4 d. AgNO3 |
front 26 In a mountain location where the atmospheric pressure is less than 1.00 atm, water will boil at ___. | back 26 a. a temperature less than 100ºC b. 100ºC c. a temperature greater than 100ºC d. None of these choices. |
front 27 How many milliliters of 6.0 M HCl must be diluted to make 500.0 mL of a 0.10 M solution? | back 27 a. 1.2 mL b. 8.3 mL c. 30. mL d. 300 mL |
front 28 The atmospheric pressure is greater at the top of the mountain than in the valley below. | back 28 True False |
front 29 A cylinder of gas originally at 28.0°C and 1.00 atm of pressure is heated to 112.0°C. What is the new pressure? | back 29 a. 4.00 atm b. 0.250 . 0.782 atm d. 1.28 atm |
front 30 Which of these contains the largest particles? | back 30 a. solution b. colloid c. suspension d. solvent |
front 31 Red blood cells will shrink in a hypotonic solution. | back 31 True False |
front 32 A patient’s blood test indicates that 1.00 mL of serum contains 0.00140 g of fibrinogen (a protein that aids in coagulation of blood). What is the concentration of the fibrinogen in mg/dL? | back 32 a. 280 mg/dL b. 14 mg/dL c. 0.014 mg/dL d. 140. mg/dL |
front 33 Blood serum has a calcium ion (Ca2+) concentration of 3.9
x 10-4
| back 33 a. 0.78 mEq/L b. 0.39 mEq/L c. 2.3 mEq/L d. 6.8 mEq/L |
front 34 If red blood cells were to be stored in a hypertonic solution in the blood bank, then | back 34 a. there would be no problems as this matches the red blood cell’s composition .b. the cells would tend to explode due to the movement of water into the cells. c. the water would flow out of the cells resulting in shrinkage and wrinkling. . there would be too much water present to store red blood cells effectively. |
front 35 In a homogeneous mixture | back 35 a. the substances are not evenly distributed. b. the substances are always liquid. c. the substances are always solids. d. the substances are uniformly distributed. |
front 36 15.0 g of KCl is dissolved into a solution with a total volume of 250.0 mL. What is the molarity of the solution? | back 36 a. 0.200 M b. 3.75 M c. 0.804 M d. 1.24 M |
front 37 How many grams of AgNO3 are required to make 500.0 mL of a 0.10 M solution? | back 37 a. 8.5 g b. 17 g c. 85 g d. 0.10 g |
front 38 A prodrug is | back 38 a. used because it is less expensive than the regular medication. b. a more professional type of medication than is normally used. c. the metabolic result of normal bodily processes on commonly available materials. d. a precursor of a drug that is converted by metabolic processes to that drug. |
front 39 A sample of neon gas fills a 6.85-L container. What is the new temperature (in Kelvin) if the gas is transferred into a 9.64-L container and pressure adjusted to 604 torr? | back 39 a. 305 K b. 483 K c. 244 K d. 656 K |
front 40 A gas occupies a volume of 8.60 L at 45C and 764 torr. What pressure would it occupy if the volume is adjusted to 13.3 L at 20C? | back 40 a. 536 torr b. 455 torr c. 109 torr d. 188 torr |
front 41 Pentose, C5H10O5, reacts with oxygen to produce carbon dioxide and water. What is the coefficient on O2 when the equation below is properly balanced? | back 41 a. 3 b. 5 c. 7 d. 15 |
front 42 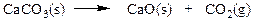 Which set of coefficients properly balance the equation below, going from reactants to products? | back 42 a. 1,2,1,4 b. 2,1,1,2 c. 2,2,3,1 d. 1,2,2,1 |
front 43 An oxidizing agent is the reactant in an oxidation - reduction reaction that causes reduction of another reactant by providing electrons for the other reactant to accept. | back 43 True False |
front 44 Balance the equation below and determine the number of moles of oxygen required to completely burn 5.00 moles of propane. | back 44 a. 5.00 b. 10.0 c. 15.0 d. 25.0 |
front 45 When ∆G for a reaction is +286 kcal, the reaction is spontaneous. | back 45 True False |
front 46 While this isn’t a real reaction, we can say that carbonic acid, H2CO3, can be produced directly from the elements. Choose the statement that is correct when the given masses are supplied to react. | back 46 a. Hydrogen is the limiting reactant. b. Carbon is the limiting reactant. c. Oxygen is the limiting reactant. d. There is no limiting reactant. |
front 47 What will you observe if you mix a solution of KOH with a solution of MgI2
| back 47 . bubbles of KI will form b. a solid of Mg(OH)2 will settle in reaction vessel c. a solid of KOH will form d. a clear solution will form |
front 48 Two methanol molecules can react in the presence of sulfuric acid to produce dimethyl ether. Calculate the percent yield for this reaction that was run in a laboratory starting with 50 grams methanol and producing 26 grams dimethyl ether. | back 48 a. 52% b. 57% c. 72% d. 200% |
front 49 What mass of potassium chloride, a salt substitute often used by heart patients, can be produced directly from 5.2 g potassium and 7.9 g chlorine? | back 49 a. 5.2 g KCl b. 4.9 g KCl c. 9.9 g KCl d. 10.1 g KCl |
front 50 Whipped cream is an example of a ___. | back 50 a. solution b. suspension c. colloid d. solvent |