Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Microbiology 1 Lab Practical 1
front 1 MPN | back 1 (Most Probable Number) indirect/viable method, live cells. Make statistical estimates numbers of cells by their patterns of growth in liq. culture media |
front 2 Standard plate count (SPC) | back 2 Direct/viable, live cells, good for patients. Dilute a sample in saline, spread on solid media & count colonies. Calculate the # of cells in original sample from counts & dilution. *most common* |
front 3 Spectroscopy | back 3 Indirect/total live & dead, not good for patients. Cells dead & alive. Measure the amount of light that passes thru a liq. culture using a spectrophotometer & est. the # of cells/mL based on amount of light that passes through the culture. * most common * |
front 4 DAPI or AODC staining & microscopy | back 4 Direct/total. Live/dead. Stain the cells w/fluorescent dyes, which make them visible in raw samples (ie soil). Count the # of cells using a fluorescent microscope. |
front 5 # of colonies needed on media to give accurate counting results | back 5 30-300 |
front 6 Spectroscopy method of counting (how it works) | back 6 based on turbidity. Optical density used to describe the turbidity of a culture. The Spec 20 can quantify the OD of a culture indirectly by measuring the amount of light that can pass through a a culture (percent transmittance %T). The higher the OD the lower the %T (indirectly proportional, the higher the OD the higher the turbidity (directly proportional) |
front 7 If you were given a stock culture that was really old, would you expect the SPC to be higher or lower? | back 7 Lower because most bacteria in death phase |
front 8 With SPC/dilution, why bother doing 3 replicates? | back 8 To normalize & standardie counts |
front 9 Why shouldn't you use a plate that has 10 colonies to calulate the number of cells in the starting culture? | back 9 Not enough to get statisical validity. NETC |
front 10 Which counting method would you use if you need isolated colonies from a soil sample and why? | back 10 DAPI or AODC staining. You can apply flourescent stain to bacteria you want to to see and use fluoresent microsope. |
front 11 Kirby-Bauer Technique | back 11 Using paper disks impregnated w/different antibacterial medicines on Mueller-Hinton agar plate to determine the sensitivity of a bacterium to serveral antibacterial medicines. No growth or halo around disk = sensitive (zone of inhabition--use whole diameter). Grows right up to the disk = resistant. |
front 12 Robert Kock | back 12 In the 1880s he developed streaking for isolation. |
front 13 Aseptic technique | back 13 Lab technique used to exclude contaminants. |
front 14 Why is it desirable that microscope objectives be parfocal? | back 14 So you can focus it once, then switch objectives and refocus only using fine adjustment knob. |
front 15 Which objective focuses closest to the slide? | back 15 100x Oil immersion |
front 16 What controls the amount of light reaching the oculars? | back 16 Iris diaphragm (opens/closes)and condensor (up/down) |
front 17 What effect does increased magnification have on the field of vision? | back 17 Raise magnification lowers field of view |
front 18 What does the "ubiquity of microorganisms" refer to ? | back 18 The concept that microorganisms are everywhere |
front 19 Fastidious organisms | back 19 Live in body and have "finicky" nutrient requirements |
front 20 What are the objective powers and names? | back 20 4x scanning, 10x low power, 40x high powerh(high dry), 100x oil immersion |
front 21 Primary purpose of: deeps, slants, broths? | back 21 Deeps= ovservie O2 requirements and motility. Slants = surface growth and pattern. Broths = large numbers of bacterial growth. |
front 22 Resolution (or resolving power) | back 22 The ability of lenses to reveal fine detail or 2 points distinctly separated. |
front 23 When using low-power lense, the iris diaphragm should be...? | back 23 Barely open so that good contrast is achieved. More light is needed w/higher magnification. |
front 24 Darkfield microscopy is valuable for observing...? | back 24 Bacterium that are not stainable with conventional stains, but can be observed in directd smears (ex. Treponema pallidum - syphilis). |
front 25 Brownian movement | back 25 Not true motility but rather the movement caused by molecules in the liquid striking an object. Vibrates or quivers. |
front 26 Shape of most bacteria grown in the lab | back 26 Cocci or rods |
front 27 What is the usual % agar and what is a lower % used for? | back 27 1.5% agar is normal. Semisolid deeps 0.5-o.7% agar can be used to determine motility. |
front 28 Slants vs. plates | back 28 Slants also provide a solid growth surface, but are easier to store and transport. |
front 29 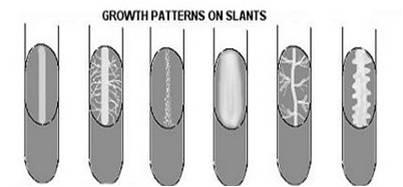 The 6 patterns of growth on agar slants | back 29 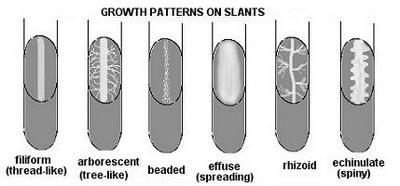 Arborescent (branched-looks like tree
|
front 30 CFU | back 30 Colony forming unit |
front 31 Where is isolation of bacteria frequently used? | back 31 On selective media |
front 32 Selective Media | back 32 Designed to encourage growth of certain types of organisms |
front 33 Differential Media | back 33 Contain indicators that expose differences between organisms (i.e. pH) |
front 34 Enteric | back 34 Gut bacteria, usually Enterobacteriaceae. Coliforms are enteros & lactosefermentators, noncoliform do not ferment lactose |
front 35 Bacteroides fragilis | back 35 The most abudnand bacteria in the human colon. 10^11 cells per gram of feces. Also the most common anerobic pathogen. Used in BBE AGar |
front 36 Growth patterns in agar & broth | back 36 -Colony shape--circular, irregular, punctiform (tiny)
|
front 37 Aerotolerance determined in what media? | back 37 Agar deep stabs and shakes |
front 38 Obligate aerobes | back 38 Require O2, grow at top |
front 39 Facultatvie anearobes | back 39 Grow with or without O2, grow throughout media but more dense on top |
front 40 Aerotolerant anaerobes | back 40 Don't require O2, but not aversely affected by it. Uniform growth throughout media |
front 41 Microaerophiles | back 41 Survive only in lower than atmospheric levels of O2, middle or upper middle growth in media. |
front 42 Capnophiles | back 42 Are microaerophiles that must have elevated CO2 |
front 43 What type of agar used in agar deep stabs? | back 43 Tryptic soy agar |
front 44 Bile salts | back 44 Used in selective media, selects against organisms incapable of surviving gut (especially gram +) |
front 45 Lactose | back 45 Helps distinguish between fermtors and nonfermentors |
front 46 Thiosulfate | back 46 Used to id organisms cable of reducing sulfure to H2S |
front 47 Ferric Iron | back 47 Inicator of sulfur reducers, black precip |