Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Experiment 9- Reactions in Aqueous Solutions: Methathesis Reactions and Net Ionic Equations
front 1 Purpose of Experiment 9 | back 1 become familiar w/ writing equations for metathesis reactions, including net ionic reactions |
front 2 Metathesis reaction model | back 2 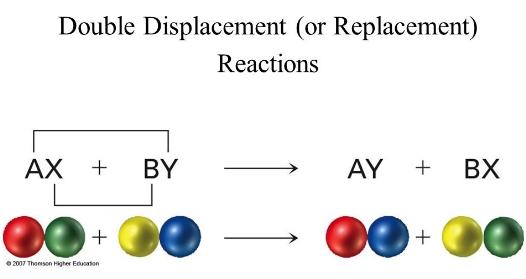 when cations and ions appear to exchange partners |
front 3 What must happen for a metathesis reaction to lead to a net change in solution? | back 3 ions must be removed from the solution |
front 4 Three driving forces for metathesis to occur | back 4 1. Formation of a precipitate 2. The formation of a weak electrolyte or nonelectrolyte 3. The formation of a gas that escapes a solution |
front 5 Typical reaction with formation of a precipitate | back 5 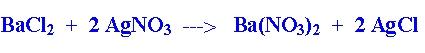 The reaction of barium chloride w/ sivler nitrate produces AgCl precipitate |
front 6 Molecular equation | back 6 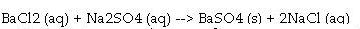 a balanced chemical equation where the ionic compounds are expressed as molecules instead of component ions |
front 7 Complete ionic equation | back 7 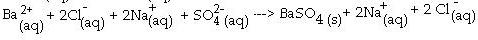 A form of writing a balanced equation in which all ions are shown |
front 8 Net ionic equation | back 8  equations that show only the soluble, strong electrolytes reacting (represented as ions) and omit the spectator ions |
front 9 spectator ions | back 9  ions that appear on both sides of a complete ionic reaction and are removed from the complete ionic equation to produce the net ionic equation these ions exists in the same form on both the reactant and product sides of a reaction. They are unchanged on both sides of a chemical equation and do not affect equilibrium. |