Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Experiment 29: Diazotization/Diazocoupling, Preparation of Methyl Orange INC
front 1 Draw two resonance structures for the nitrosonium ion, NO+. | back 1 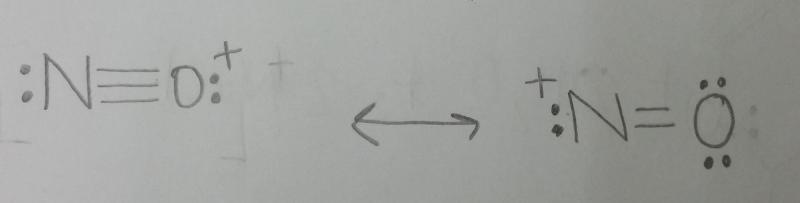 |
front 2 What is the equation for generating NO+ in a dilute solution of HCl and NaONO? | back 2 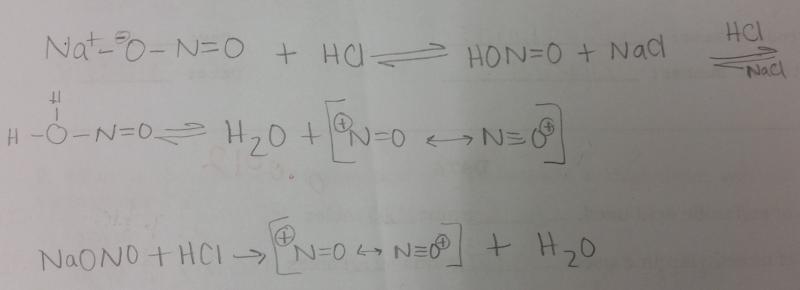 |
front 3 What is the balanced equation for the reaction between sulfanilic acid and sodium carbonate? | back 3 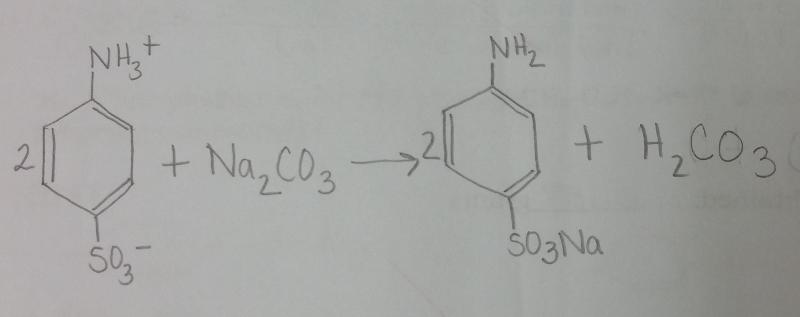 |
front 4 What is the balanced equation for the reaction of sodium sulfanilate, NaONO, and HCl? | back 4 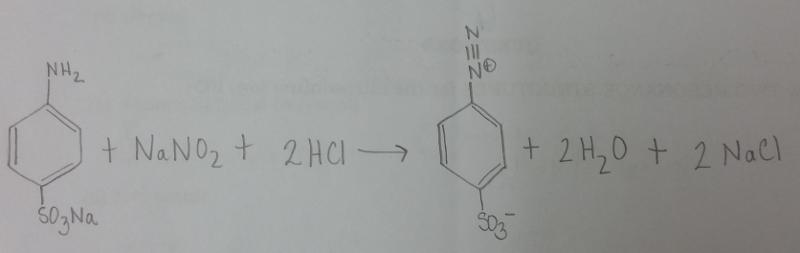 |
front 5 What is the balanced equation for the reaction between diazonium salt and N,N,-dimethylaniline? | back 5 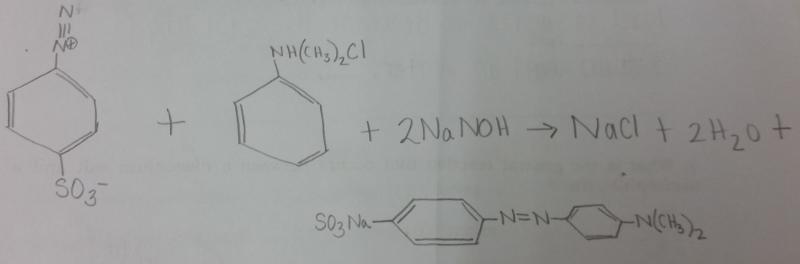 |
front 6 Show by using arrows the movement of electron pairs when the diazonium ion, attacking as an electrophile, attacks N,N,-dimethylaniline. The product of the attack is resonance-stabalized aromatic carbocation. | back 6 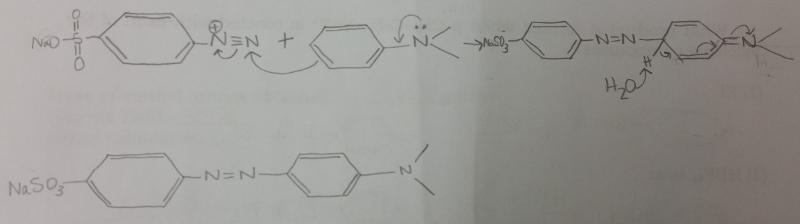 |
front 7 What is the purpose of adding NaOH to the red crystals of methyl orange? | back 7 NaOH is added to ensure that the methyl orange is alkaline. It also neutralizes the HCl so a salt can form. |
front 8 Why is NaCl added to the reaction mixture before final crystallization? | back 8 NaCl is added to decrease the solubility of the sodium salt in water. |
front 9 What is the general reaction that occurs between diazonium salt and a nucleophile, Nu-? | back 9 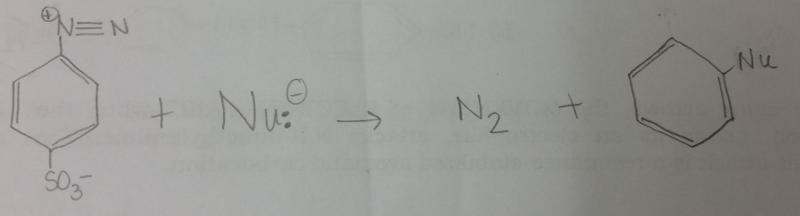 |
front 10 What is the product between diazonium and KI? | back 10 |
front 11 What is the product between diazonium and HBF4 and heat? | back 11  |
front 12 What is the product between diazonium and CuCN, then H2 catalyst? | back 12 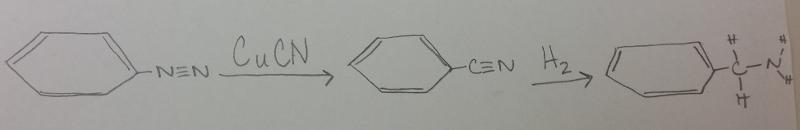 |
front 13 What is the product between diazonium and phenol, then H2 catalyst? | back 13  |
front 14 What is the product between diazonium and o-methylphenol (o-cresol), then H2 catalyst? | back 14 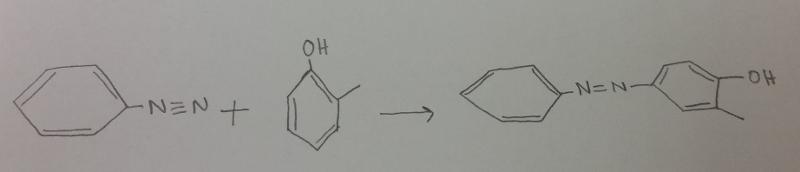 |
front 15 What is the product between diazonium H+, and water? | back 15  |
front 16 benzene diazonium | back 16 no data |
front 17 diazonium | back 17 no data |