Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Bio 1441 - Final Study Guide
front 1 What factors are most important in determining which elements are
most common in living matter? | back 1 Answer: E |
front 2 Why is each element unique and different from other elements in
chemical properties? | back 2 Answer: C |
front 3 Knowing just the atomic mass of an element allows inferences about
which of the following? | back 3 Answer: D |
front 4 Electrons exist only at fixed levels of potential energy. However, if
an atom absorbs sufficient energy, a possible result is that | back 4 Answer: A |
front 5 Which of the following explains most specifically the attraction of
water molecules to one another? | back 5 Answer: D |
front 6 In the term trace element, the modifier trace means that | back 6 Answer: A |
front 7 Van der Waals interactions result when | back 7 Answer: B |
front 8 Which of the following correctly describes chemical equilibrium?
| back 8 Answer: A |
front 9 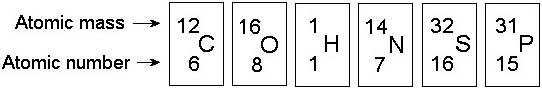 In the figure above, how many electrons does nitrogen have in its valence shell? A) 2 | back 9 Answer: B |
front 10 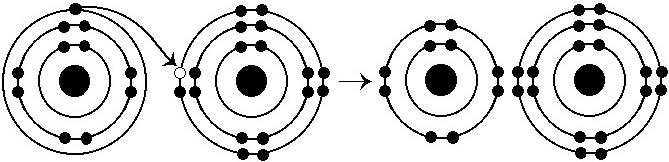 What results from the chemical reaction illustrated above? | back 10 Answer: E |
front 11 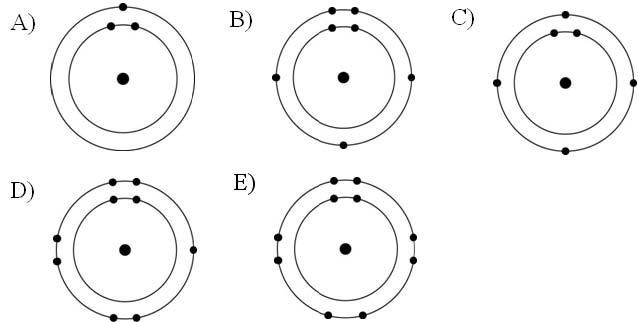 Which one of the atoms shown would be most likely to form an anion with a charge of -1? | back 11 Answer: D |
front 12 Compared with ³¹P, the radioactive isotope ³²P has | back 12 Answer: E |
front 13 About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living matter?
| back 13 Answer: D |
front 14 Liquid water's high specific heat is mainly a consequence of the
| back 14 Answer: C |
front 15 Which type of bond must be broken for water to vaporize? | back 15 Answer: D |
front 16 Why does ice float in liquid water? | back 16 Answer: D |
front 17 You have a freshly prepared 0.1 M solution of glucose in water. Each
liter of this solution contains how many glucose molecules? | back 17 Answer: E |
front 18 What is the pH of a solution with a hydroxyl ion [OH⁻] concentration
of 10⁻¹² M? | back 18 Answer: A |
front 19 If the pH of a solution is decreased from 9 to 8, it means that the
| back 19 Answer: E |
front 20 How would acidification of seawater affect marine organisms? | back 20 Answer: D |
front 21 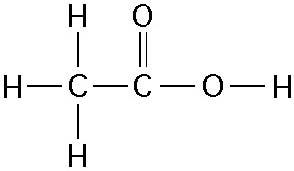 How many grams of the compound in the figure above would be required
to make 1 L of a 0.5 M solution? | back 21 Answer: B |
front 22 The partial negative charge in a molecule of water occurs because
| back 22 Answer: B |
front 23 Which of the following effects is produced by the high surface
tension of water? | back 23 Answer: B |
front 24 Hydrophobic substances such as vegetable oil are | back 24 Answer: A |
front 25 What is the hydrogen ion [H⁺] concentration of a solution of pH 8?
| back 25 Answer: D |
front 26 If the pH of a solution is increased from pH 5 to pH 7, it means that
the | back 26 Answer: C |
front 27 The element present in all organic molecules is | back 27 Answer: C |
front 28 Hermann Kolbe's synthesis of an organic compound, acetic acid, from
inorganic substances that had been prepared directly from pure
elements was a significant milestone for what reason? | back 28 Answer: E |
front 29 Which of the following statements correctly describes cis-trans
isomers? | back 29 Answer: A |
front 30 Compared to a hydrocarbon chain where all the carbon atoms are linked
by single bonds, a hydrocarbon chain with the same number of carbon
atoms, but with one or more double bonds, will | back 30 Answer: B |
front 31 Organic molecules with only hydrogens and five carbon atoms can have
different structures in all of the following ways except | back 31 Answer: E |
front 32 Which two functional groups are always found in amino acids? A) ketone and methyl | back 32 Answer: C |
front 33 Testosterone and estradiol are | back 33 Answer: B |
front 34 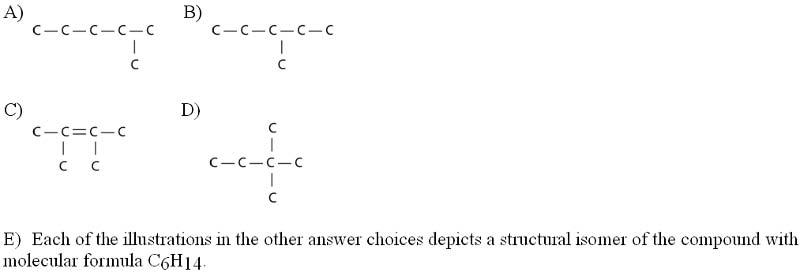 Three or four of the following illustrations depict different structural isomers of the organic compound with molecular formula C₆H₁₄. For clarity, only the carbon skeletons are shown; hydrogen atoms that would be attached to the carbons have been omitted. Which one, if any, is NOT a structural isomer of this compound? | back 34 Answer: C |
front 35 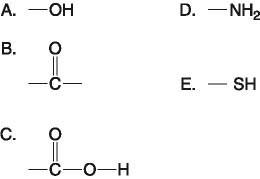 Which functional group(s) shown above is (are) present in all amino
acids? | back 35 Answer: E |
front 36 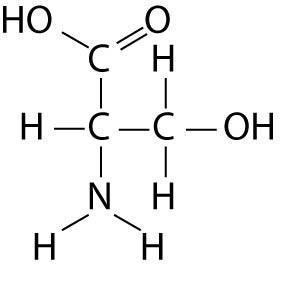 Which functional group is not present in this molecule? | back 36 Answer: B |
front 37 Why are hydrocarbons insoluble in water? | back 37 Answer: B |
front 38 Research indicates that ibuprofen, a drug used to relieve inflammation and pain, is a mixture of two enantiomers; that is, molecules that A) have identical chemical formulas but differ in the branching
of their carbon skeletons. | back 38 Answer: B |
front 39 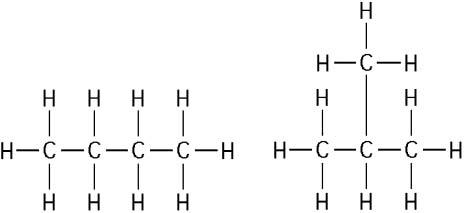 The two molecules shown in the figure above are best described as
| back 39 Answer: C |
front 40 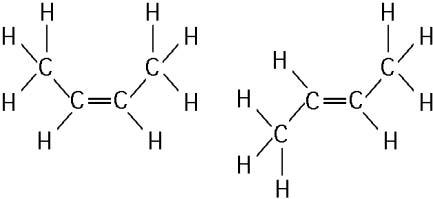 The two molecules shown in the figure above are best described as A) enantiomers. | back 40 Answer: E |
front 41 Which of these molecules is not formed by dehydration reactions?
| back 41 Answer: A |
front 42 Which of the following is not a polymer? | back 42 Answer: A |
front 43 On food packages, to what does the term insoluble fiber refer? | back 43 Answer: A |
front 44 All of the following contain amino acids except | back 44 Answer: B |
front 45 What aspects of protein structure are stabilized or assisted by
hydrogen bonds? | back 45 Answer: E |
front 46 Which bonds are created during the formation of the primary structure
of a protein? | back 46 Answer: A |
front 47 What maintains the secondary structure of a protein? | back 47 Answer: B |
front 48 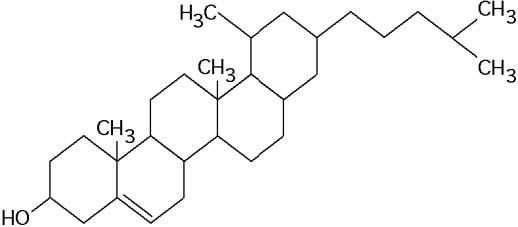 What is the structure shown in the figure?
| back 48 Answer: C |
front 49 Which class of biological polymer has the greatest functional
variety? | back 49 Answer: B |
front 50 The structural level of a protein least affected by a disruption in
hydrogen bonding is the | back 50 Answer: A |
front 51 Which of the following statements is true for the class of biological
molecules known as lipids? | back 51 Answer: A |
front 52 There are 20 different amino acids. What makes one amino acid
different from another? | back 52 Answer: C |
front 53 Dehydration reactions are used in forming which of the following
compounds? | back 53 Answer: E |
front 54 The volume enclosed by the plasma membrane of plant cells is often
much larger than the corresponding volume in animal cells. The most
reasonable explanation for this observation is that | back 54 Answer: C |
front 55 Large numbers of ribosomes are present in cells that specialize in
producing which of the following molecules? | back 55 Answer: C |
front 56 A cell with a predominance of free ribosomes is most likely | back 56 Answer: B |
front 57 Hydrolytic enzymes must be segregated and packaged to prevent general
destruction of cellular components. Which of the following organelles
contains these hydrolytic enzymes in animal cells? | back 57 Answer: B |
front 58 One of the key innovations in the evolution of eukaryotes from a
prokaryotic ancestor is the endomembrane system. What eukaryotic
organelles or features might have evolved as a part of, or as an
elaboration of, the endomembrane system? | back 58 Answer: D |
front 59 If an individual has abnormal microtubules, due to a hereditary
condition, in which organs or tissues would you expect dysfunction?
| back 59 Answer: D |
front 60 The cell walls of bacteria, fungi, and plant cells and the
extracellular matrix of animal cells are all external to the plasma
membrane. Which of the following is a characteristic common to all of
these extracellular structures? | back 60 Answer: D |
front 61 A mutation that disrupts the ability of an animal cell to add
polysaccharide modifications to proteins would most likely cause
defects in its | back 61 Answer: D |
front 62 In a liver cell detoxifying alcohol and some other poisons, the
enzymes of the peroxisome remove hydrogen from these molecules and
| back 62 Answer: D |
front 63 The extracellular matrix is thought to participate in the regulation
of animal cell behavior by communicating information from the outside
to the inside of the cell via which of the following? | back 63 Answer: D |
front 64 Plasmodesmata in plant cells are most similar in function to which of the following structures in animal cells? A) peroxisomes | back 64 Answer: C |
front 65 What types of proteins are not synthesized in the rough ER? | back 65 Answer: D |
front 66 Which type of organelle is found in plant cells but not in animal
cells? | back 66 Answer: D |
front 67 Which of the following is one of the ways that the membranes of
winter wheat are able to remain fluid when it is extremely cold?
| back 67 Answer: A |
front 68 Water passes quickly through cell membranes because | back 68 Answer: E |
front 69 A bacterium engulfed by a white blood cell through phagocytosis will
be digested by enzymes contained in | back 69 Answer: B |
front 70 In the small airways of the lung, a thin layer of liquid is needed
between the epithelial cells and the mucus layer in order for cilia to
beat and move the mucus and trapped particles out of the lung. One
hypothesis is that the volume of this airway surface liquid is
regulated osmotically by transport of sodium and chloride ions across
the epithelial cell membrane. How would the lack of a functional
chloride channel in cystic fibrosis patients affect sodium ion
transport and the volume of the airway surface liquid? | back 70 Answer: C |
front 71 A protein that spans the phospholipid bilayer one or more times is
| back 71 Answer: A |
front 72 What kinds of molecules pass through a cell membrane most easily?
| back 72 Answer: B |
front 73 Which of the following would likely move through the lipid bilayer of
a plasma membrane most rapidly? | back 73 Answer: A |
front 74 Which of the following statements is correct about diffusion? | back 74 Answer: C |
front 75 Mammalian blood contains the equivalent of 0.15 M NaCl. Seawater
contains the equivalent of 0.45 M NaCl. What will happen if red blood
cells are transferred to seawater? | back 75 Answer: A |
front 76 When a plant cell, such as one from a peony stem, is submerged in a
very hypotonic solution, what is likely to occur? | back 76 Answer: E |
front 77 Glucose diffuses slowly through artificial phospholipid bilayers. The
cells lining the small intestine, however, rapidly move large
quantities of glucose from the glucose-rich food into their
glucose-poor cytoplasm. Using this information, which transport
mechanism is most probably functioning in the intestinal cells? | back 77 Answer: E |
front 78 The sodium-potassium pump is called an electrogenic pump because it
| back 78 Answer: C |
front 79 The movement of potassium into an animal cell requires | back 79 Answer: C |
front 80 Which term most precisely describes the cellular process of breaking
down large molecules into smaller ones? | back 80 Answer: E |
front 81 Which of the following is (are) true for anabolic pathways? | back 81 Answer: C |
front 82 Which of the following is a statement of the first law of
thermodynamics? | back 82 Answer: A |
front 83 Living organisms increase in complexity as they grow, resulting in a
decrease in the entropy of an organism. How does this relate to the
second law of thermodynamics? | back 83 Answer: D |
front 84 Which of the following types of reactions would decrease the entropy
within a cell? | back 84 Answer: A |
front 85 The mathematical expression for the change in free energy of a system
is ΔG =ΔH - TΔS. Which of the following is (are) correct? | back 85 Answer: C |
front 86 When ATP releases some energy, it also releases inorganic phosphate.
What purpose does this serve (if any) in the cell? | back 86 Answer: D |
front 87 Reactants capable of interacting to form products in a chemical
reaction must first overcome a thermodynamic barrier known as the
reaction's | back 87 Answer: B |
front 88 How does a noncompetitive inhibitor decrease the rate of an enzyme
reaction? | back 88 Answer: B |
front 89 Succinate dehydrogenase catalyzes the conversion of succinate to
fumarate. The reaction is inhibited by malonic acid, which resembles
succinate but cannot be acted upon by succinate dehydrogenase.
Increasing the ratio of succinate to malonic acid reduces the
inhibitory effect of malonic acid. | back 89 Answer: A |
front 90 If an enzyme in solution is saturated with substrate, the most
effective way to obtain a faster yield of products is to | back 90 Answer: A |
front 91 Which of the following is the smallest closed system? | back 91 Answer: E |
front 92 A system at chemical equilibrium | back 92 Answer: E |
front 93 Which of the following statements describes NAD⁺? | back 93 Answer: A |
front 94 Why are carbohydrates and fats considered high energy foods? | back 94 Answer: D |
front 95 In glycolysis, for each molecule of glucose oxidized to pyruvate
| back 95 Answer: B |
front 96 What is proton-motive force? | back 96 Answer: B |
front 97 In liver cells, the inner mitochondrial membranes are about five
times the area of the outer mitochondrial membranes. What purpose must
this serve? | back 97 Answer: C |
front 98 Which statement best supports the hypothesis that glycolysis is an
ancient metabolic pathway that originated before the last universal
common ancestor of life on Earth? | back 98 Answer: A |
front 99 What is the purpose of beta oxidation in respiration? | back 99 Answer: E |
front 100 The immediate energy source that drives ATP synthesis by ATP synthase
during oxidative phosphorylation is the | back 100 Answer: D |
front 101 Which metabolic pathway is common to both fermentation and cellular
respiration of a glucose molecule? | back 101 Answer: C |
front 102 The final electron acceptor of the electron transport chain that
functions in aerobic oxidative phosphorylation is | back 102 Answer: A |
front 103 When electrons flow along the electron transport chains of
mitochondria, which of the following changes occurs? | back 103 Answer: A |
front 104 Which process in eukaryotic cells will proceed normally whether
oxygen (O₂) is present or absent? | back 104 Answer: B |
front 105 A molecule that is phosphorylated | back 105 Answer: D |
front 106 In any ecosystem, terrestrial or aquatic, what group(s) is (are)
always necessary? | back 106 Answer: D |
front 107 In autotrophic bacteria, where are the enzymes located that can carry
on carbon fixation (reduction of carbon dioxide to carbohydrate)?
| back 107 Answer: C |
front 108 A plant has a unique photosynthetic pigment. The leaves of this plant
appear to be reddish yellow. What wavelengths of visible light are
being absorbed by this pigment? | back 108 Answer: B |
front 109 Assume a thylakoid is somehow punctured so that the interior of the
thylakoid is no longer separated from the stroma. This damage will
have the most direct effect on which of the following processes?
| back 109 Answer: D |
front 110 In photosynthetic cells, synthesis of ATP by the chemiosmotic
mechanism occurs during | back 110 Answer: C |
front 111 In a plant leaf, the reactions that produce NADH occur in | back 111 Answer: D |
front 112 Which of the following statements best represents the relationships
between the light reactions and the Calvin cycle? | back 112 Answer: A |
front 113 A spaceship is designed to support animal life for a multiyear voyage
to the outer planets of the solar system. Plants will be grown to
provide oxygen and to recycle carbon dioxide. | back 113 Answer: C |
front 114 Where does the Calvin cycle take place? | back 114 Answer: A |
front 115 What are the products of linear photophosphorylation? | back 115 Answer: C |
front 116 For anaphase to begin, which of the following must occur? | back 116 Answer: C |
front 117 At the M phase checkpoint, the complex allows for what to occur?
| back 117 Answer: A |
front 118 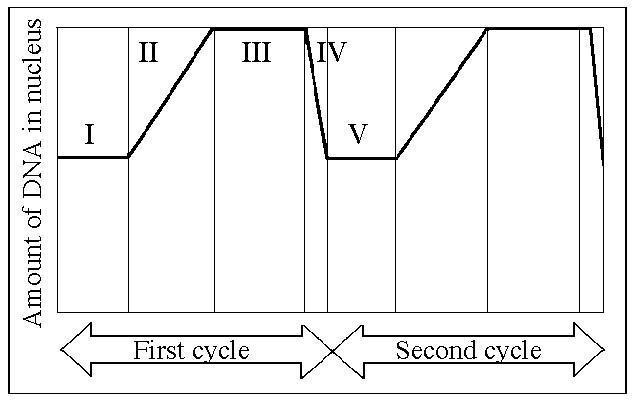 Which number represents DNA synthesis? | back 118 Answer: B |
front 119 Reduction of oxygen to form water occurs during | back 119 Answer: B |
front 120 The reactions that produce molecular oxygen (O₂) take place in | back 120 Answer: A |
front 121 What is the primary function of the Calvin cycle? | back 121 Answer: E |
front 122 The centromere is a region in which | back 122 Answer: A |
front 123 Which of the following describe(s) cyclin-dependent kinase (Cdk)?
| back 123 Answer: E |
front 124 Which of the following most accurately describes a cyclin? | back 124 Answer: D |
front 125 Nucleotides can be radiolabeled before they are incorporated into
newly forming DNA and can therefore be assayed to track their
incorporation. In a set of experiments, a student—faculty research
team used labeled T nucleotides and introduced these into the culture
of dividing human cells at specific times. | back 125 Answer: B |
front 126 Nucleotides can be radiolabeled before they are incorporated into newly forming DNA and can therefore be assayed to track their incorporation. In a set of experiments, a student—faculty research team used labeled T nucleotides and introduced these into the culture of dividing human cells at specific times. The research team used the setup to study the incorporation of
labeled nucleotides into a culture of lymphocytes and found that the
lymphocytes incorporated the labeled nucleotide at a significantly
higher level after a pathogen was introduced into the culture. They
concluded that | back 126 Answer: C |
front 127 One difference between cancer cells and normal cells is that cancer
cells | back 127 Answer: C |
front 128 Which of the following does not occur during mitosis? | back 128 Answer: B |
front 129 A particular cell has half as much DNA as some other cells in a
mitotically active tissue. The cell in question is most likely in
| back 129 Answer: A |
front 130 At which phase are centrioles beginning to move apart in animal
cells? | back 130 Answer: E |
front 131 Where do the microtubules of the spindle originate during mitosis in
both plant and animal cells? | back 131 Answer: B |
front 132 In the human species, all somatic cells have 46 chromosomes. Which of
the following can also be true? | back 132 Answer: A |
front 133 The human X and Y chromosomes | back 133 Answer: D |
front 134 Which of the following is true of a species that has a chromosome
number of 2n = 16? | back 134 Answer: C |
front 135 Referring to a plant's sexual life cycle, which of the following
terms describes the process that leads directly to the formation of
gametes? | back 135 Answer: B |
front 136 Which of the following best describes a karyotype? | back 136 Answer: B |
front 137 In a human karyotype, chromosomes are arranged in 23 pairs. If we
choose one of these pairs, such as pair 14, which of the following do
the two chromosomes of the pair have in common? | back 137 Answer: C |
front 138 A cell divides to produce two daughter cells that are genetically
different. | back 138 Answer: B |
front 139 Independent assortment of chromosomes occurs. | back 139 Answer: B |
front 140 A tetrad includes which of the following sets of DNA strands? | back 140 Answer: B |
front 141 To visualize and identify meiotic cells at metaphase with a
microscope, what would you look for? | back 141 Answer: E |
front 142 For the following question, match the key event of meiosis with the stages listed below. Tetrads of chromosomes are aligned at the equator of the spindle;
alignment determines independent assortment. | back 142 Answer: B |
front 143 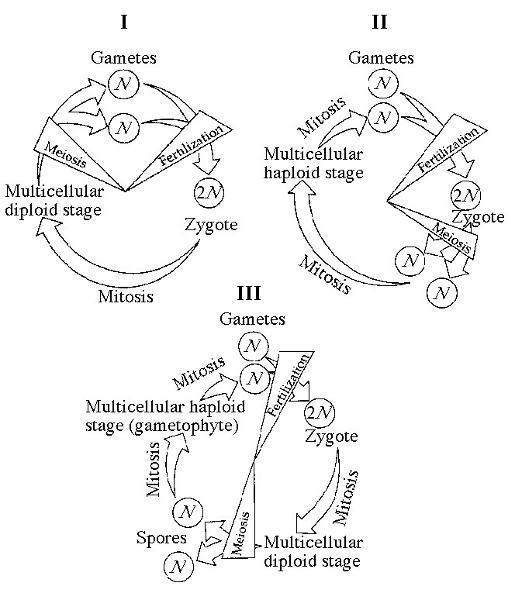 Which of the life cycles is typical for most fungi and some protists?
| back 143 Answer: B |
front 144 After telophase I of meiosis, the chromosomal makeup of each daughter
cell is | back 144 Answer: D |
front 145 Why did Mendel continue some of his experiments to the F₂ or F₃
generation? | back 145 Answer: B |
front 146 Cystic fibrosis affects the lungs, the pancreas, the digestive
system, and other organs, resulting in symptoms ranging from breathing
difficulties to recurrent infections. Which of the following terms
best describes this? | back 146 Answer: C |
front 147 Hydrangea plants of the same genotype are planted in a large flower
garden. Some of the plants produce blue flowers and others pink
flowers. This can be best explained by which of the following? | back 147 Answer: E |
front 148 Which of the following provides an example of epistasis? | back 148 Answer: C |
front 149 How could you best predict the maximum number of alleles for a single
gene whose polypeptide product is known? | back 149 Answer: E |
front 150 One of two major forms of a human condition called neurofibromatosis
(NF 1) is inherited as a dominant gene, although it may range from
mildly to very severely expressed. If a young child is the first in
her family to be diagnosed, which of the following is the best
explanation? | back 150 Answer: B |
front 151 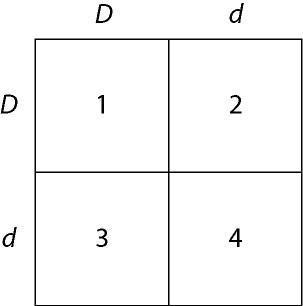 In a particular plant, leaf color is controlled by gene locus D.
Plants with at least one allele D have dark green leaves, and plants
with the homozygous recessive dd genotype have light green leaves. A
true-breeding dark-leaved plant is crossed with a light-leaved one,
and the F₁ offspring is allowed to self-pollinate. The predicted
outcome of the F₂ is diagrammed in the Punnett square shown in Figure
14.1, where 1, 2, 3, and 4 represent the genotypes corresponding to
each box within the square. | back 151 Answer: E |
front 152 Two true-breeding stocks of pea plants are crossed. One parent has
red, axial flowers and the other has white, terminal flowers; all F₁
individuals have red, axial flowers. The genes for flower color and
location assort independently. | back 152 Answer: B |
front 153 Two true-breeding stocks of pea plants are crossed. One parent has red, axial flowers and the other has white, terminal flowers; all F₁ individuals have red, axial flowers. The genes for flower color and location assort independently. Among the F₂ offspring, what is the probability of plants with
white axial flowers? | back 153 Answer: C |
front 154 Marfan syndrome in humans is caused by an abnormality of the
connective tissue protein fibrillin. Patients are usually very tall
and thin, with long spindly fingers, curvature of the spine, sometimes
weakened arterial walls, and sometimes ocular problems, such as lens
dislocation. Which of the following would you conclude about Marfan
syndrome from this information? | back 154 Answer: D |
front 155 When crossing an organism that is homozygous recessive for a single
trait with a heterozygote, what is the chance of producing an
offspring with the homozygous recessive phenotype? | back 155 Answer: C |
front 156 Which of the following describes the ability of a single gene to have
multiple phenotypic effects? | back 156 Answer: C |
front 157 Which of the following is an example of polygenic inheritance? | back 157 Answer: E |
front 158 Which of the following is the meaning of the chromosome theory of
inheritance as expressed in the early 20th century? | back 158 Answer: B |
front 159 Thomas Hunt Morgan's choice of Drosophila melanogaster has been
proven to be useful even today. Which of the following has/have
continued to make it a most useful species? | back 159 Answer: E |
front 160 Calico cats are female because | back 160 Answer: B |
front 161 Duchenne muscular dystrophy (DMD) is caused by a gene on the human X
chromosome. The patients have muscles that weaken over time because
they have absent or decreased dystrophin, a muscle protein. They
rarely live past their 20s. How likely is it for a woman to have this
condition? | back 161 Answer: D |
front 162 Which of the following statements is true of linkage? | back 162 Answer: A |
front 163 Why does recombination between linked genes continue to occur? | back 163 Answer: C |
front 164 Map units on a linkage map cannot be relied upon to calculate
physical distances on a chromosome for which of the following reasons?
| back 164 Answer: A |
front 165 A phenotypically normal prospective couple seeks genetic counseling
because the man knows that he has a translocation of a portion of his
chromosome 4 that has been exchanged with a portion of his chromosome
12. Although he is normal because his translocation is balanced, he
and his wife want to know the probability that his sperm will be
abnormal. What is your prognosis regarding his sperm? | back 165 Answer: A |
front 166 Which of the following is true of aneuploidies in general? A) A monosomy is more frequent than a trisomy. | back 166 Answer: B |
front 167 Mitochondrial DNA is primarily involved in coding for proteins needed
for electron transport. Therefore, in which body systems would you
expect most mitochondrial gene mutations to be exhibited? | back 167 Answer: D |
front 168 Sex determination in mammals is due to the SRY region of the Y
chromosome. An abnormality of this region could allow which of the
following to have a male phenotype? | back 168 Answer: B |
front 169 An inversion in a human chromosome often results in no demonstrable
phenotypic effect in the individual. What else may occur? | back 169 Answer: B |
front 170 What is the source of the extra chromosome 21 in an individual with
Down syndrome? | back 170 Answer: D |
front 171 Cytosine makes up 42% of the nucleotides in a sample of DNA from an
organism. Approximately what percentage of the nucleotides in this
sample will be thymine? | back 171 Answer: A |
front 172 Which of the following can be determined directly from X-ray
diffraction photographs of crystallized DNA? | back 172 Answer: A |
front 173 In an analysis of the nucleotide composition of DNA, which of the
following will be found? | back 173 Answer: C |
front 174 Replication in prokaryotes differs from replication in eukaryotes for
which of the following reasons? | back 174 Answer: B |
front 175 Which enzyme catalyzes the elongation of a DNA strand in the 5' → 3'
direction? | back 175 Answer: C |
front 176 Polytene chromosomes of Drosophila salivary glands each consist of
multiple identical DNA strands that are aligned in parallel arrays.
How could these arise? | back 176 Answer: B |
front 177 To repair a thymine dimer by nucleotide excision repair, in which
order do the necessary enzymes act? | back 177 Answer: E |
front 178 Which of the following would you expect of a eukaryote lacking
telomerase? | back 178 Answer: D |
front 179 Use the following list of choices for the following question | back 179 Answer: E |
front 180 Which of the following statements describes chromatin? | back 180 Answer: C |
front 181 What is the function of topoisomerase? | back 181 Answer: A |
front 182 What is the role of DNA ligase in the elongation of the lagging
strand during DNA replication? | back 182 Answer: C |
front 183 Use the following list of choices for the following question | back 183 Answer: D |
front 184 Use the following list of choices for the following question I. helicase | back 184 Answer: A |
front 185 Use the following list of choices for the following question | back 185 Answer: C |
front 186 Which of the following sets of materials are required by both
eukaryotes and prokaryotes for replication? | back 186 Answer: A |