Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Introductions to Chemistry Chapter 5
front 1 True or False:
| back 1 False |
front 2 Which of the following applies to the proton?
| back 2 B) charge = +1
|
front 3 True or False:
| back 3 False |
front 4 True or False:
| back 4 True |
front 5 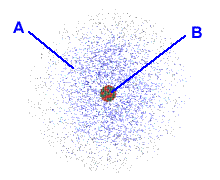 Choose the region of the atom that accounts for most of its mass | back 5 B |
front 6 How many electrons, protons, ans neutrons are there in an atom of tin-117, ^117^Sn? | back 6 Protons: 50
|
front 7 Write the composition of one atom of the ^119^Sn isotope. (electrons, protons, ans neutrons) | back 7 Protons: 50
|
front 8 Write the composition of one atom of the ^28^Si isotope. (electrons, protons, ans neutrons) | back 8 Protons: 14
|
front 9 Fill in the rest;
| back 9 Element Symbol: Cl
|
front 10 Fill in the rest;
| back 10 Element Symbol: Given
|
front 11 Fill in the rest;
| back 11 Element Symbol: Cl
|
front 12 How many protons, neutrons and electrons are there in a neutral atom of the isotope with the nuclear symbol:
| back 12 protons:26
|
front 13 How many protons, neutrons and electrons are there in a neutral atom of phosphorus-28? | back 13 protons: 15
|
front 14 a) What is the mass number of an atom that contains 7 protons, 7 neutrons, and 7 electrons?
| back 14 a) mass number = 14
|
front 15 a) How many protons and neutrons are there in the nucleus of an atom that has an atomic number of 11 and a mass number of 20?
| back 15 a) protons = 11 neutrons = 9
|
front 16 A certain element consists of two stable isotopes.
| back 16 69.72 amu |
front 17 The element antimony has two stable isotopes, antimony-121 with an atomic mass of 120.9038 amu and antimony-123 with an atomic mass of 122.9041 amu. From the atomic mass of Sb = 121.75 one can conclude that:
| back 17 C. antimony-121 has the highest percent natural abundance |
front 18 A certain element consists of two stable isotopes.
| back 18 121.76 amu |
front 19 a) What is the symbol for the element that is in group 5A and period 3?
| back 19 a) P
|
front 20 Refer to a periodic table. Give the symbol for an element that is:
| back 20 1) Sc
|
front 21 Refer to a periodic table. Which of the following elements are nonmetals?
| back 21 A & C |