Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Bonding
front 1 What is the formula for the compound formed by calcium and nitrogen?
| back 1 D |
front 2 What is the best description of the carbon-oxygen bond lengths in CO32–?
| back 2 C |
front 3 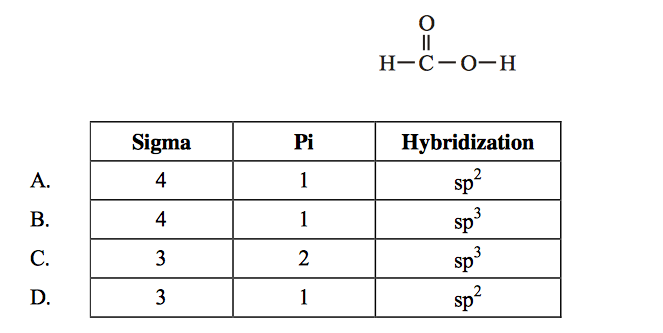 What is the number of sigma () and pi () bonds and the hybridization of the carbon atom in: | back 3 A |
front 4 Element X is in group 2, and element Y in group 7, of the periodic table. Which ions will be present in the compound formed when X and Y react together?
| back 4 B |
front 5 Which of the following increase(s) for the bonding between carbon atoms in the sequence of
| back 5 B |
front 6 Which of the following contain a bond angle of 90°?
| back 6 C |
front 7 Which allotropes contain carbon atoms with sp2 hybridization?
| back 7 C |
front 8 Based on electronegativity values, which bond is the most polar?
| back 8 B |
front 9  What is the Lewis (electron dot) structure for sulfur dioxide? | back 9 D |
front 10 Which substance is most soluble in water (in mol dm–3) at 298 K?
| back 10 C |
front 11 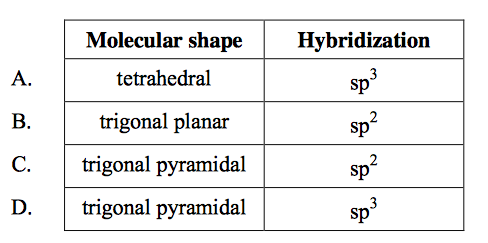 What is the molecular shape and the hybridization of the nitrogen atom in NH3? | back 11 D |
front 12 Which statement about sigma and pi bonds is correct?
| back 12 C |
front 13 According to VSEPR theory, repulsion between electron pairs in a valence shell decreases in the order
| back 13 A |
front 14 Which molecule is linear?
| back 14 B |
front 15 Why is the boiling point of PH3 lower than that of NH3?
| back 15 B |
front 16 Which molecule is non-polar?
| back 16 B |
front 17 Consider the following statements.
| back 17 B |
front 18 What happens when sodium and oxygen combine together?
| back 18 B |
front 19 Which statement is correct about two elements whose atoms form a covalent bond with each other?
| back 19 B |
front 20 In ethanol, C2H5OH (l), there are covalent bonds, hydrogen bonds and van der Waals’ forces. Which bonds or forces are broken when ethanol is vaporized?
| back 20 D |
front 21 Which substance has the lowest electrical conductivity?
| back 21 C |
front 22 Which statement best describes the attraction present in metallic bonding?
| back 22 B |
front 23 Which statement is correct about multiple bonding between carbon atoms?
| back 23 D |
front 24 When the following bond types are listed in decreasing order of strength (strongest first), what is the correct order?
| back 24 A |
front 25 Which statement is true for most ionic compounds?
| back 25 D |
front 26 What is the valence shell electron pair repulsion (VSEPR) theory used to predict?
| back 26 B |
front 27 Which fluoride is the most ionic?
| back 27 B |
front 28 Which particles can act as ligands in complex ion formation?
| back 28 D |
front 29 Which statements correctly describe the NO2– ion?
| back 29 B |
front 30 30. Which substance is most similar in shape to NH3?
| back 30 D |
front 31 31. Which statement is a correct description of electron loss in this reaction? 2Al + 3S -> Al2S3
| back 31 B |
front 32 Which molecule has the smallest bond angle?
| back 32 B |
front 33 In which substance is hydrogen bonding present?
| back 33 D |
front 34 Which is a correct description of metallic bonding?
| back 34 C |
front 35 Which is the smallest bond angle in the PF5 molecule?
| back 35 A |
front 36 Which types of hybridization are shown by the carbon atoms in the compound CH2 = CH-CH3?
| back 36 C |
front 37 What intermolecular forces are present in gaseous hydrogen?
| back 37 D |
front 38 Which molecule is polar?
| back 38 B |
front 39 What are responsible for the high electrical conductivity of metals?
| back 39 B |
front 40 Which compound has the least covalent character?
| back 40 D |
front 41 Identify the types of hybridization shown by the carbon atoms in the molecule CH3CH2CH2COOH:
| back 41 C |
front 42 When C2H4, C2H2 and C2H6 are arranged in order of increasing C–C bond length, what is the correct order?
| back 42 C |
front 43 Which compound contains both ionic and covalent bonds?
| back 43 D |
front 44  | back 44 B |
front 45 Which species has a trigonal planar shape?
| back 45 A |
front 46 When C2H4, C2H2 and C2H6 are arranged in order of increasing C–C bond length, what is the correct order?
| back 46 C |
front 47 Which molecule is square planar in shape?
| back 47 B |
front 48 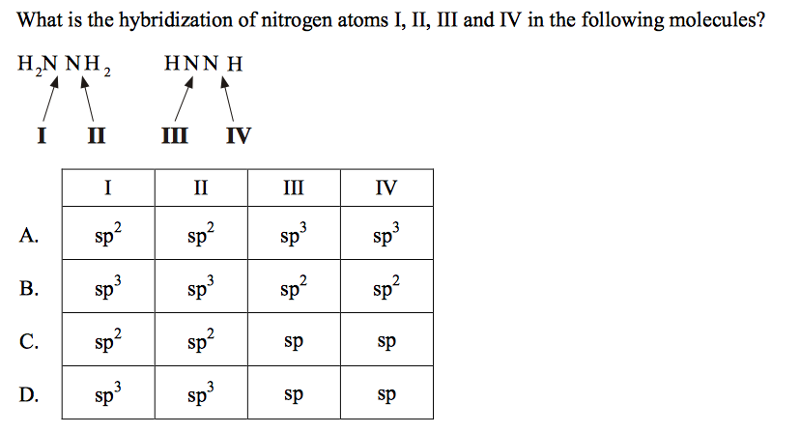 | back 48 B |
front 49 What is the formula for an ionic compound formed between an element, X, from group 2 and an element, Y, from group 6?
| back 49 A |
front 50 In the molecules N2H4, N2H2, and N2, the nitrogen atoms are linked by single, double and triple bonds, respectively. When these molecules are arranged in increasing order of the lengths of their nitrogen to nitrogen bonds (shortest bond first) which order is correct?
| back 50 D |
front 51 The compounds listed have very similar molar masses. Which has the strongest intermolecular forces?
| back 51 B |
front 52 52. What is the shape of the CO32– ion and the approximate O–C–O bond angle?
| back 52 C |
front 53 What is the molecular geometry and the Cl–I–Cl bond angle in the ICl4– ion? A. Square planar, 90
| back 53 A |
front 54 What is the geometry of the bonds around an atom with sp2 hybridization? A. 2 bonds at 180
| back 54 B |
front 55 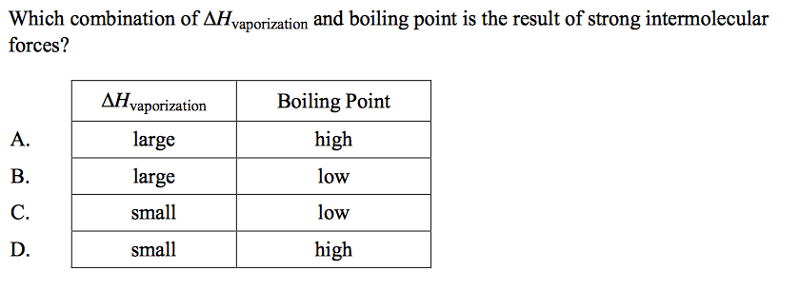 | back 55 A |
front 56 What is the formula of the compound formed when aluminium reacts with oxygen?
| back 56 B |
front 57 Which statement is true for compounds containing only covalent bonds?
| back 57 D |
front 58 How many electrons are used in the carbon-carbon bond in C2H2?
| back 58 A |
front 59 Which compound has the highest boiling point?
| back 59 B |
front 60 60. What type of solid materials are typically hard, have high melting points and poor electrical conductivities?
| back 60 B |
front 61 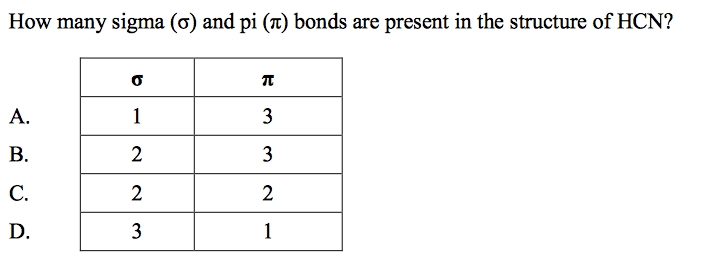 | back 61 C |
front 62 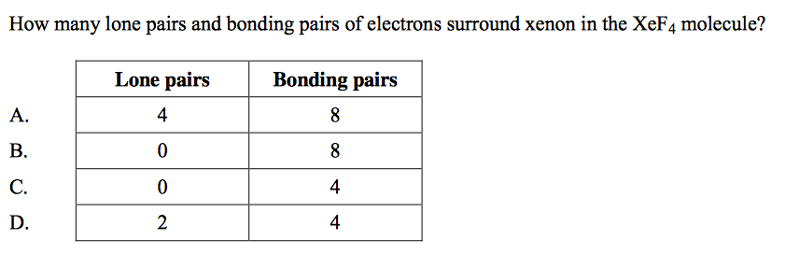 | back 62 D |