Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Atomic
front 1 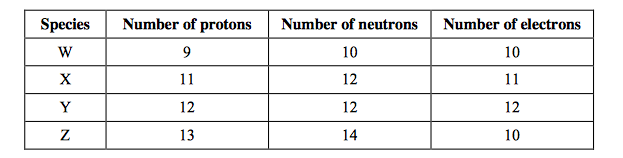 Consider the composition of the species W, X, Y and Z below. Which species is an anion?
| back 1 A |
front 2 Energy levels for an electron in a hydrogen atom are
| back 2 B |
front 3 Which is related to the number of electrons in the outer main energy level of the elements from the alkali metals to the halogens?
| back 3 A |
front 4 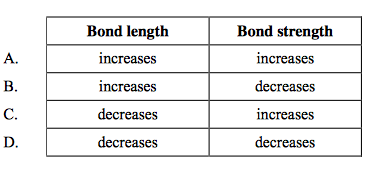 How do bond length and bond strength change as the number of bonds between two atoms increases? | back 4 C |
front 5 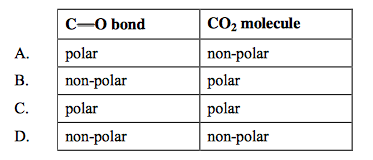 Which of the following is true for CO2? | back 5 A |
front 6 The molar masses of C2H6, CH3OH and CH3F are very similar. How do their boiling points compare?
| back 6 D |
front 7 What is the electron configuration for an atom with Z = 22?
| back 7 D |
front 8 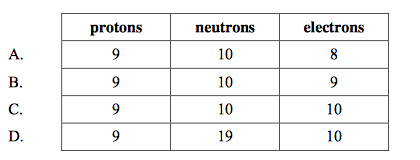 What is the correct number of each particle in a fluoride ion, 19F–? | back 8 C |
front 9 Which statement is correct for the emission spectrum of the hydrogen atom?
| back 9 D |
front 10 Which is the correct description of polarity in F2 and HF molecules?
| back 10 D |
front 11 Which types of bonding are present in CH3CHO in the liquid state?
| back 11 A |
front 12 Which statement(s) is/are generally true about the melting points of substances?
| back 12 B |
front 13 What is the correct sequence for the processes occurring in a mass spectrometer?
| back 13 A |
front 14 How many valence electrons are present in an atom of an element with atomic number 16?
| back 14 C |
front 15 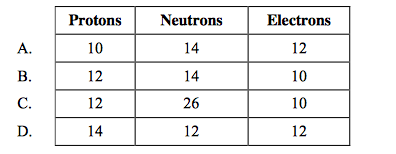 How many protons, neutrons and electrons are there in the species 26Mg2+? | back 15 B |
front 16 What is the total number of p orbitals containing one or more electrons in germanium (atomic number 32)?
| back 16 D |
front 17 A certain sample of element Z contains 60% of 69Z and 40% of 71Z. What is the relative atomic mass of element Z in this sample?
| back 17 B |
front 18 What is the difference between two neutral atoms represented by the symbols 59 Co and 59 Ni?
| back 18 D |
front 19 A certain sample of element Z contains 60% of 69Z and 40% of 71Z. What is the relative atomic mass of element Z in this sample?
| back 19 B |
front 20 Which ion would undergo the greatest deflection in a mass spectrometer?
| back 20 B |
front 21 How many electrons are there in one
| back 21 A |
front 22 The electron arrangement of sodium is 2.8.1. How many occupied main electron energy levels are there in an atom of sodium?
| back 22 B |
front 23 How many electrons are there in all the d orbitals in an atom of xenon?
| back 23 C |
front 24 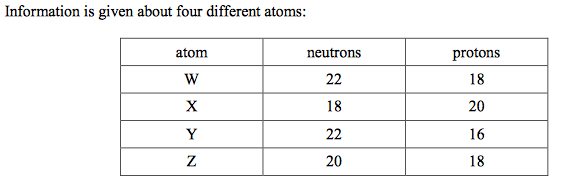 Which two atoms are isotopes?
| back 24 B |
front 25 Which statement is correct about a line emission spectrum?
| back 25 D |
front 26 How many neutrons are there in the ion 18O2–?
| back 26 B |
front 27 What is the electron arrangement of silicon?
| back 27 C |
front 28 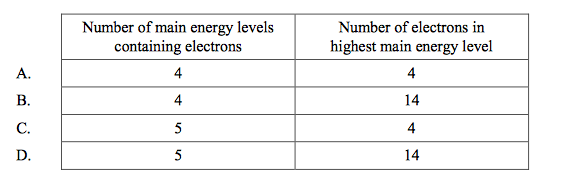 Which is correct about the element tin (Sn) (Z = 50)? | back 28 C |
front 29 Which statement is correct about the isotopes of an element?
| back 29 B |
front 30 What is the total number of electrons in p orbitals in an atom of iodine?
| back 30 D |
front 31  What is the difference between two neutral atoms represented by the symbols
| back 31 D |
front 32 Which statements are correct for the emission spectrum of the hydrogen atom?
| back 32 C |
front 33 A transition metal ion X2+ has the electronic configuration [Ar]3d9. What is the atomic number of the element?
| back 33 C |
front 34 What is the symbol for a species that contains 15 protons, 16 neutrons and 18 electrons?
| back 34 D |
front 35 What is the electron arrangement of an Al3+ ion?
| back 35 A |
front 36 What will happen to the volume of a fixed mass of gas if the pressure and the Kelvin temperature are both doubled?
| back 36 A |
front 37 How many orbitals are there in the n = 3 level of an atom?
| back 37 D |
front 38 you can do it... | back 38 A |
front 39 What is the correct sequence for the processes occurring in a mass spectrometer?
| back 39 A |
front 40 What is the electron configuration for the copper(I) ion, (Z = 29)?
| back 40 D |